Phosphorus silicate yellow-green long afterglow glass and preparing method
A phosphosilicate and long afterglow technology, which is applied in the field of phosphosilicate yellow-green long afterglow glass and its preparation, can solve the problems of low terbium ion doping concentration, complicated process, poor long afterglow performance of glass, etc., to achieve Good doping performance and luminescence performance, increase the doping concentration, and accurately control the effect of doping amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
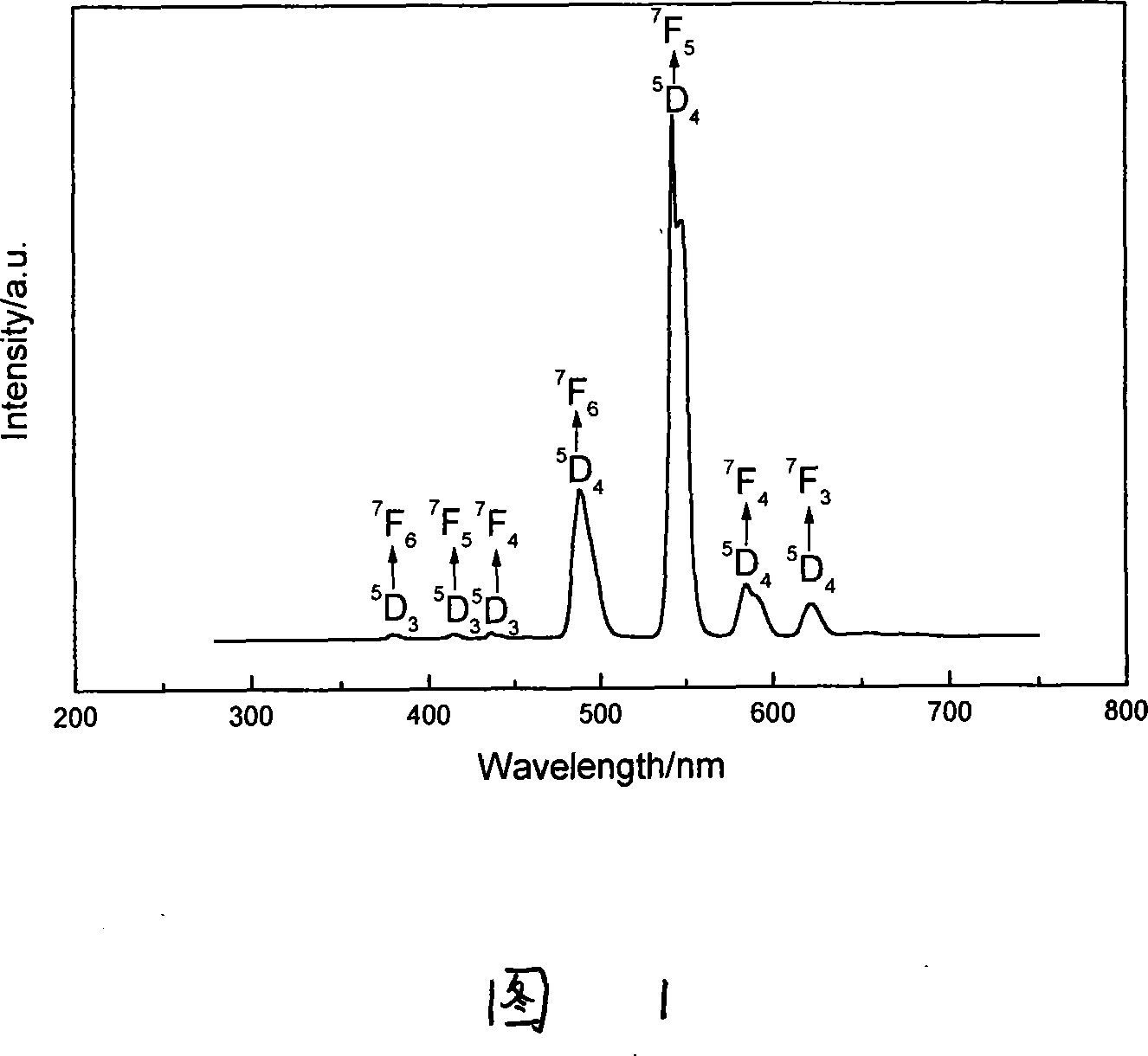
[0024] The molar percentage composition of the glass is: 73% zinc oxide, 10% phosphorus pentoxide, 15% silicon dioxide, 2% calcium oxide, and 1.25% terbium oxide. Dissolve ethyl orthosilicate in an appropriate amount of ethanol, ammonium dihydrogen phosphate and citric acid with twice the molar amount of metal ions are dissolved in distilled water, zinc oxide and calcium oxide are respectively dissolved in 1:1 nitric acid, terbium oxide is dissolved in Concentrated nitric acid, and then mix the above liquids under the condition of stirring, adjust the pH=1 after stirring for 1 hour, continue stirring for 1 hour to form a sol, put it in a water bath at 80°C for sol-gel reaction for 10 hours, and form a gel. Dry it in an oven at 100°C, heat it at 1400°C for 2 hours, and anneal it at 450°C for half an hour to obtain a colorless, transparent long afterglow glass. Excite the glass with UV254nm ultraviolet rays for half an hour, remove the excitation light source, and the glass emit...
Embodiment 2
[0026] The molar percentage composition of the glass is: 65% zinc oxide, 10% phosphorus pentoxide, 10% silicon dioxide, 5% magnesium oxide, 0.5% terbium oxide. Dissolve ethyl orthosilicate in an appropriate amount of ethanol, ammonium dihydrogen phosphate and citric acid in equimolar amounts to metal ions are dissolved in distilled water, zinc oxide and magnesium oxide are respectively dissolved in 1:1 nitric acid, terbium oxide is dissolved in concentrated Nitric acid, then mix the above liquids under the condition of stirring, adjust the pH=0.5 after stirring for 1 hour, continue to stir for 1 hour to form a sol, put it into a 90°C water bath for 8 hours through sol-gel reaction, and form a gel. Dry in an oven at 100°C, react at 1400°C for 1 hour, and anneal at 450°C for 2 hours to obtain a colorless, transparent long afterglow glass. Excite the glass with UV254nm ultraviolet rays for half an hour, remove the excitation light source, the glass emits a bright yellow-green lon...
Embodiment 3
[0028] The molar percentage composition of the glass is: 70% zinc oxide, 20% phosphorus pentoxide, 10% silicon dioxide, and 0.25% terbium oxide. Dissolve ethyl orthosilicate in an appropriate amount of ethanol, ammonium dihydrogen phosphate and citric acid in equimolar amounts to metal ions are dissolved in distilled water, zinc oxide is dissolved in 1:1 nitric acid, terbium oxide is dissolved in concentrated nitric acid, and then Mix the above liquids under the condition of stirring, adjust the pH=1 after stirring for 1 hour, continue to stir for 1 hour to form a sol, put it in a 50°C water bath for 16 hours to form a gel, and put it in a 100°C oven to dry Dry, react at 1200°C for 0.5 hours, and anneal at 450°C for 1 hour to obtain a colorless, transparent long afterglow glass. Excite the glass with UV254nm ultraviolet rays for half an hour, remove the excitation light source, the glass emits a bright yellow-green long afterglow, and after stopping the excitation for 8 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com