Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35results about "Preparation from ureas" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
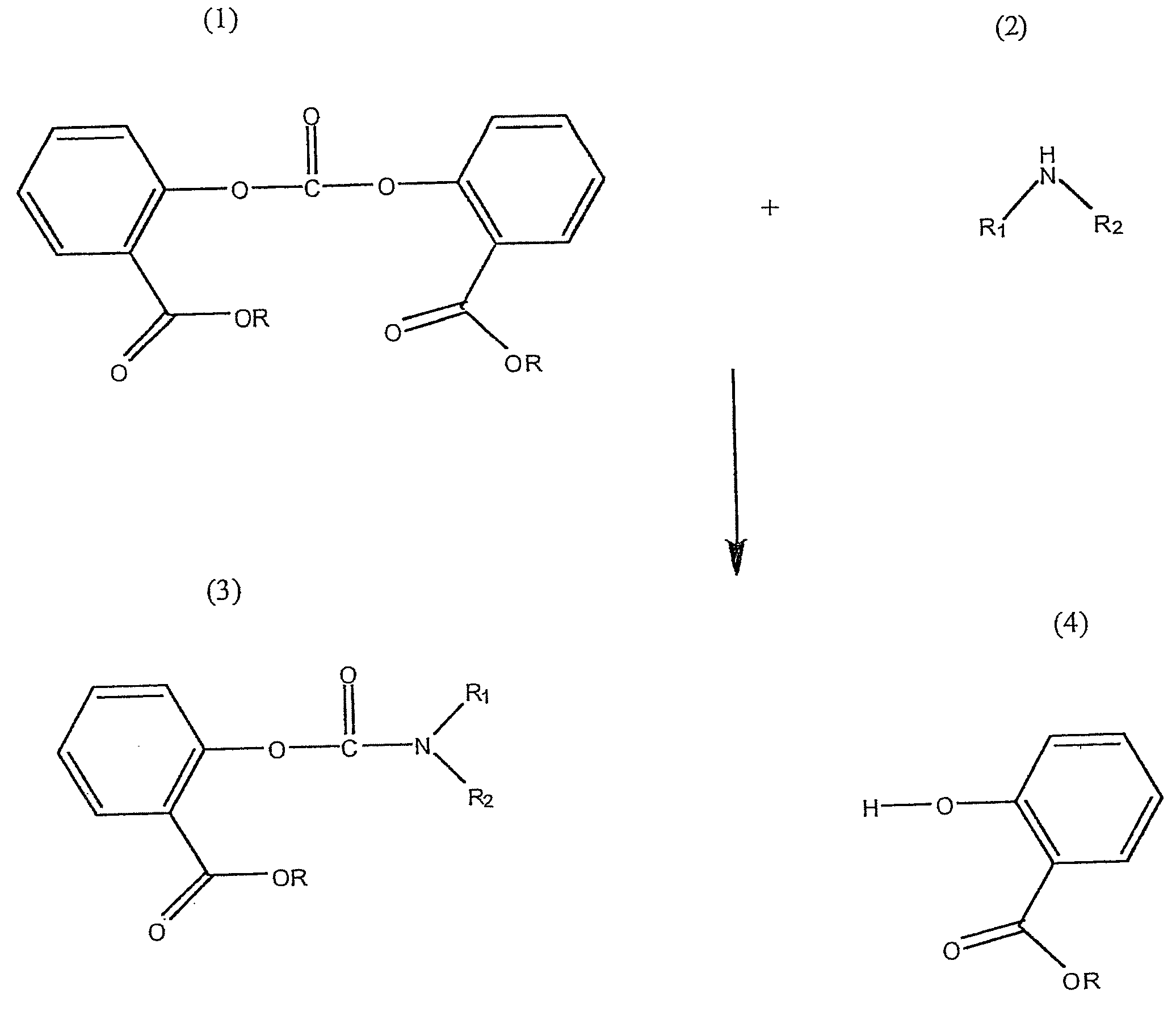
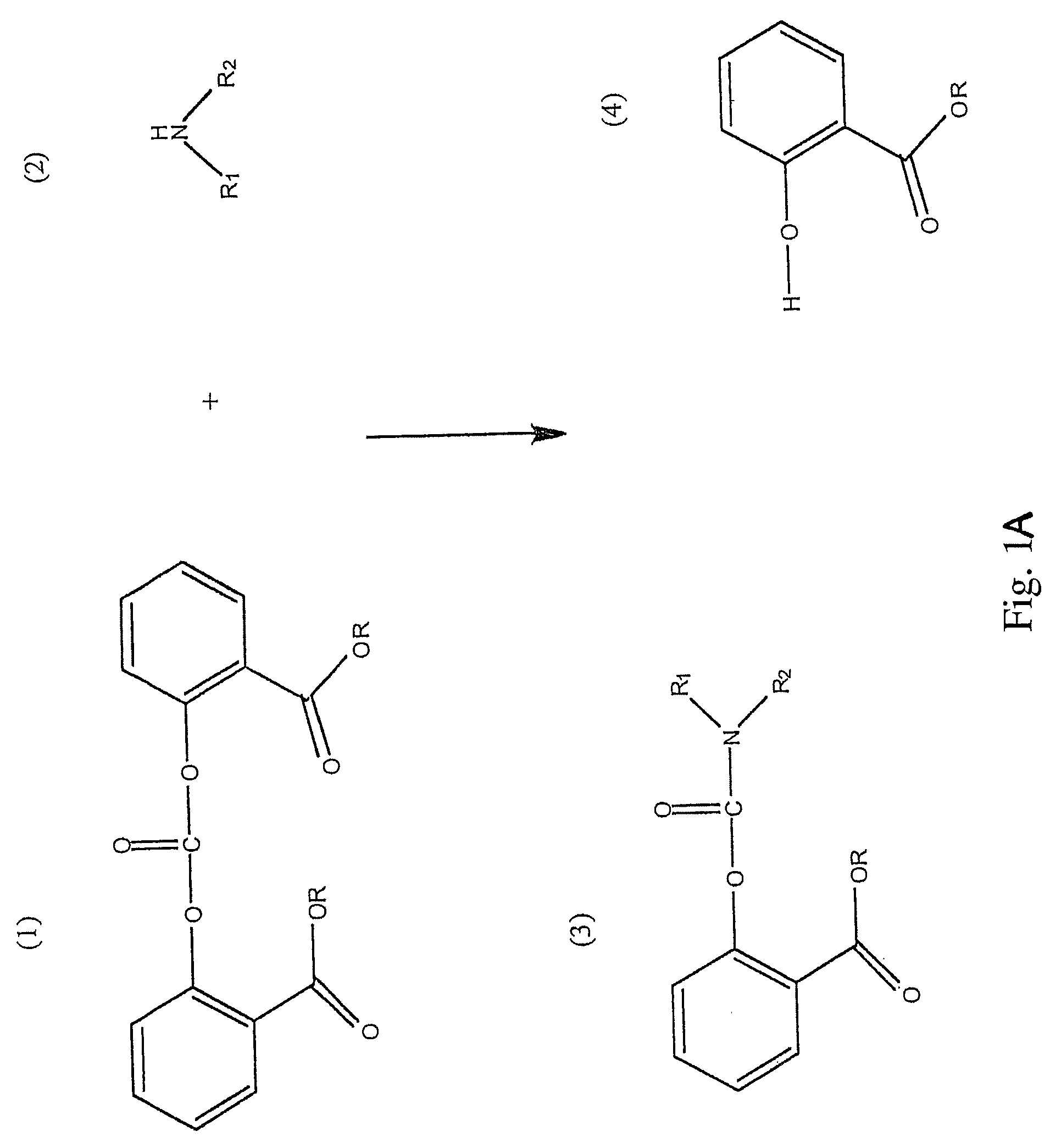
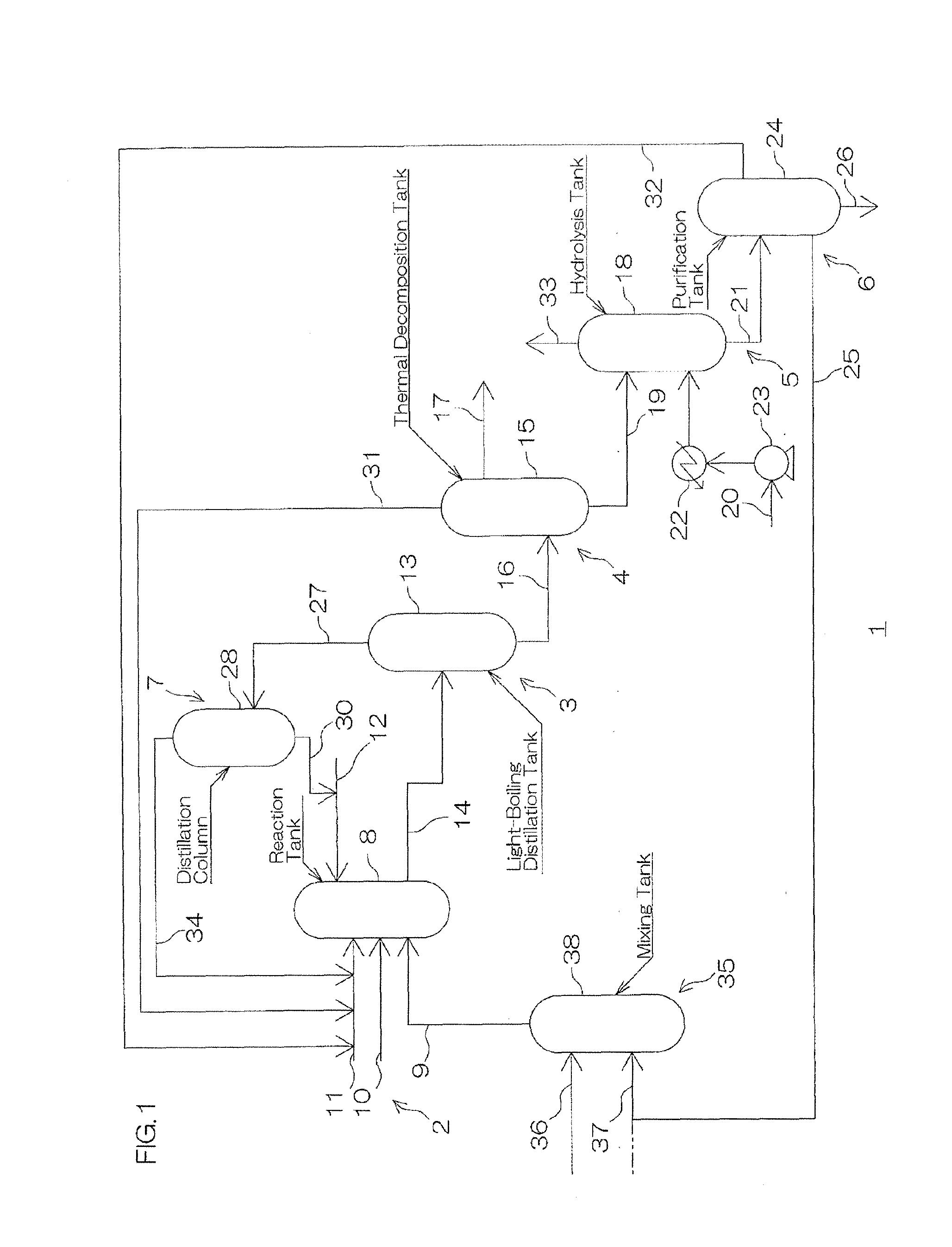
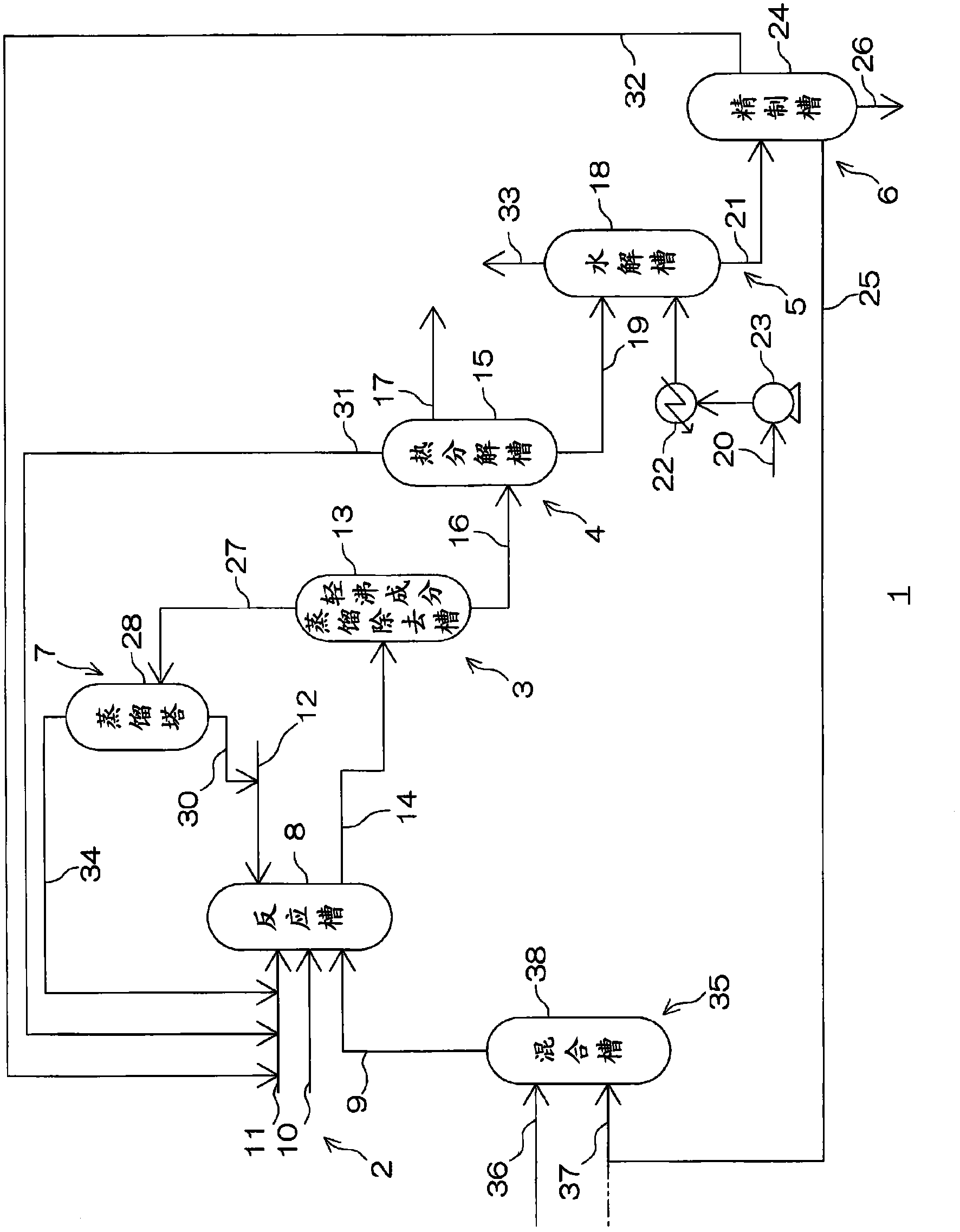
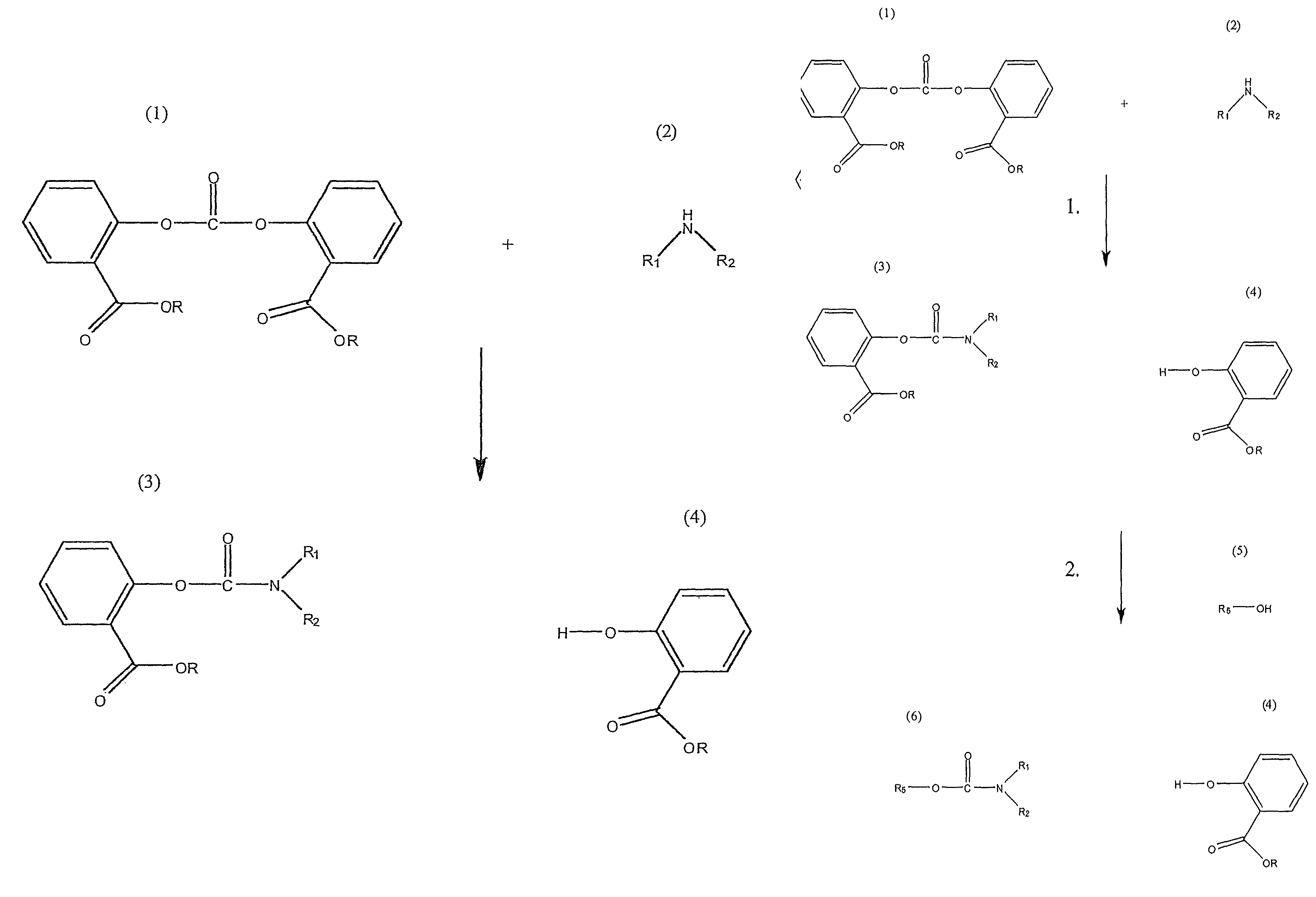
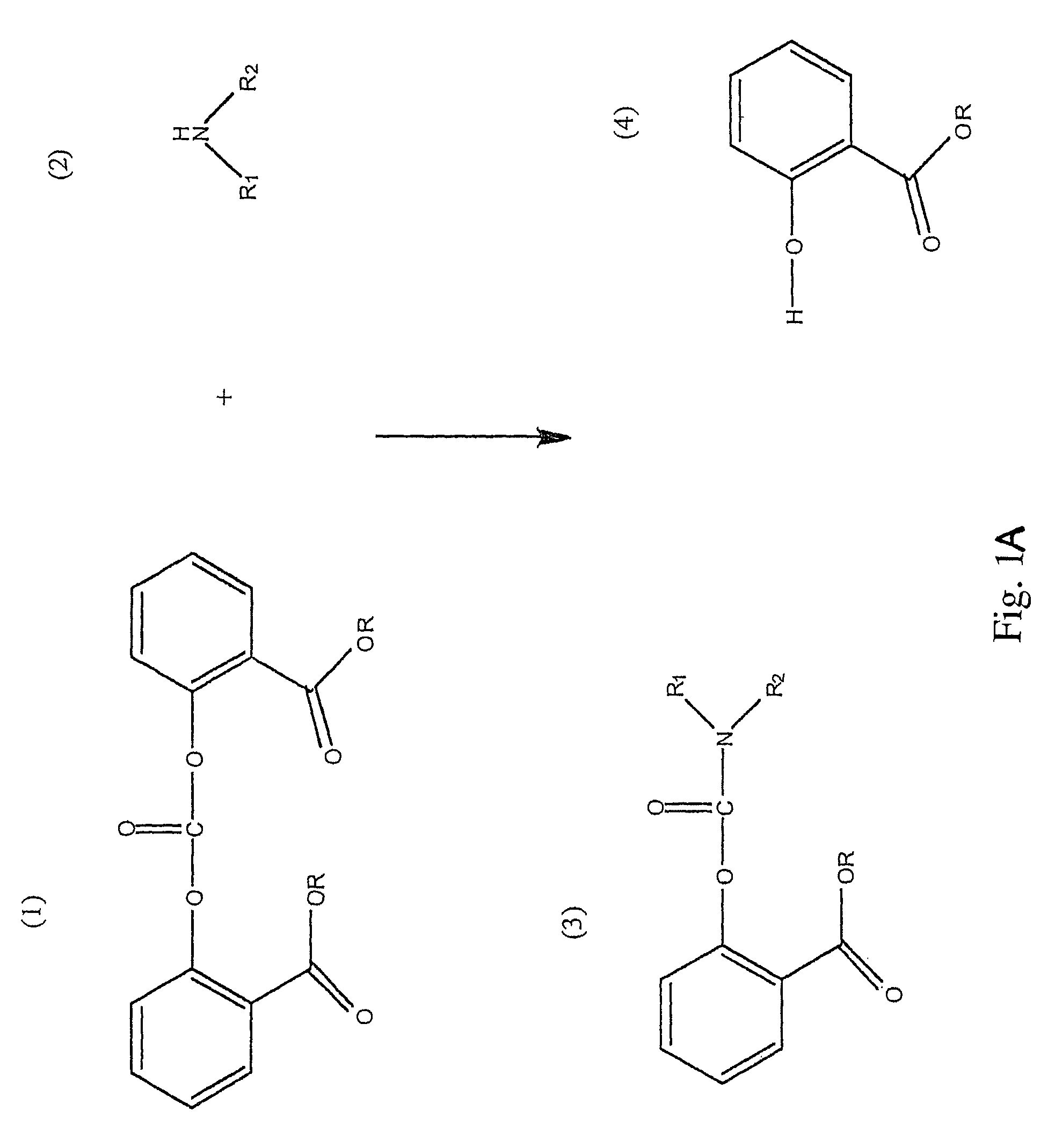
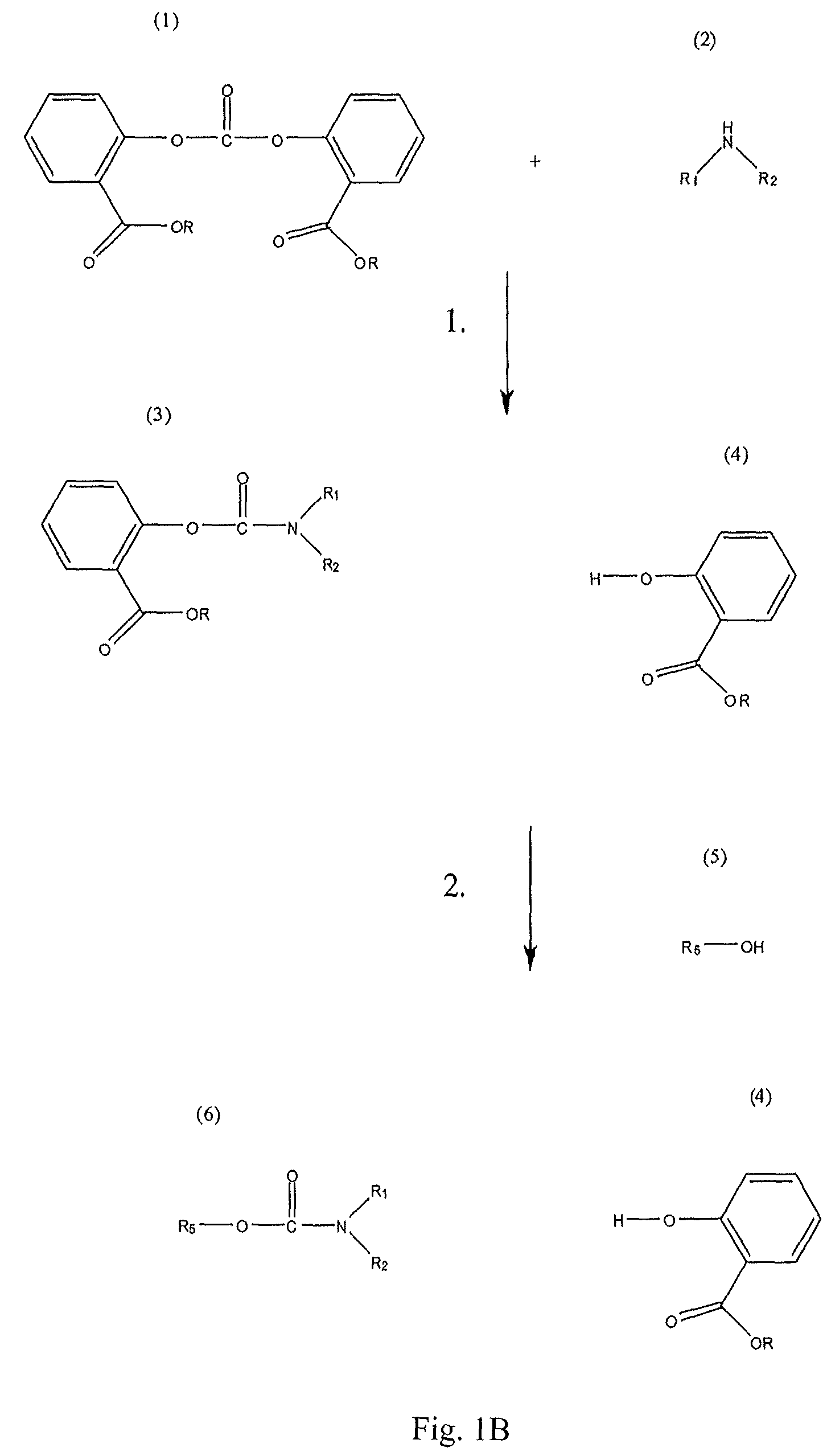
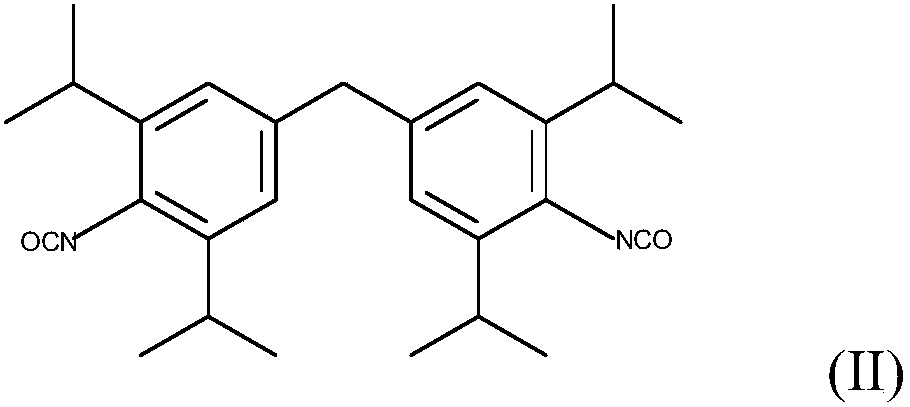
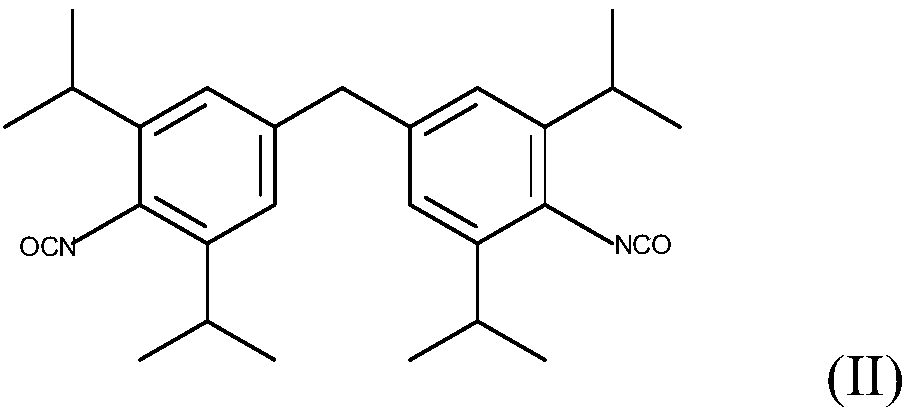
Method for Making Carbamates, Ureas and Isocyanates
InactiveUS20100113819A1Improve production yieldShort timeUrea derivatives preparationCarbamic acid derivatives preparationCarbamatePyrolysis
The present invention provides methods of forming carbamates, ureas, and isocyanates. In certain embodiments these methods include the step of reacting an amine with an ester-substituted diaryl carbonate to form an activated carbamate which can be further derivitized to form non-activated carbamate or a urea. The urea or carbamate can be subjected to a pyrolysis reaction to form isocyanate.
Owner:SABIC GLOBAL TECH BV
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS20050043563A1Speed up the processAvoid disadvantagesOrganic compound preparationPreparation from ureasPolymer sciencePhosgene
The invention relates to a multistage process for continuous and phosgene-free preparation of cycloaliphatic diisocyanates.
Owner:DEGUSSA AG
Multistage continuous preparation of cycloaliphatic diisocyanates
Cycloaliphatic diisocyanates can be prepared continuously and phosgene-free by reacting at least one cycloaliphatic diamine with at least one carbonic acid derivative and at least one alcohol to give a cycloaliphatic diurethane; freeing the cycloaliphatic diurethane of a low boiler, a medium boiler and mixtures thereof; thermally cleaving the cycloaliphatic diurethane in a cleavage apparatus to give a cycloaliphatic diisocyanate and a cleavage residue; continuously discharging a portion of the cleavage residue from the cleavage apparatus; removing the high boiler components from the discharge to obtain a purified discharge; reurethanizing the purified discharge with alcohol to obtain a reurethanized discharge; and recycling the reurethanized discharge into the process.
Owner:EVONIK DEGUSSA GMBH
Process for producing bromo chloro isocyanuric acid and its use
This invention relates to a manufacturing technique and its use of chlorine bromine isocyanuric acid. Urea and cyanamide are cyclized to cyanuric acid, and then be made into cyanuric acid suspension. Sodium hypochlorite and sodium hypobromite are added to make chlorination and bromination reaction. Chlorine bromine isocyanuric acid is got after filtration, scrubbing to filter cake, srying of the resultant. The compound of chlorine bromine isocyanuric acid and light fertilizer can be used to produce agriculture chemicals fungicide. The sodium hypochlorite and sodium hypobromite are added with no order, so its technique is convenient to control. Comparing to exist technique using chlorine and bromine gas, the sodium hypochlorite and sodium hypobromite used are liquor and hard to evaporate. The equipment request is not high, and the equipment cost can be reduced greatly, mass production cost also can be greatly reduced.
Owner:JIANGSU DONGBAO AGROCHEM
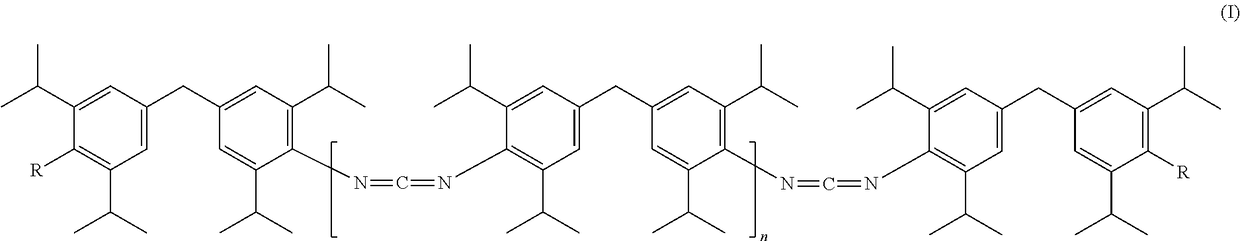
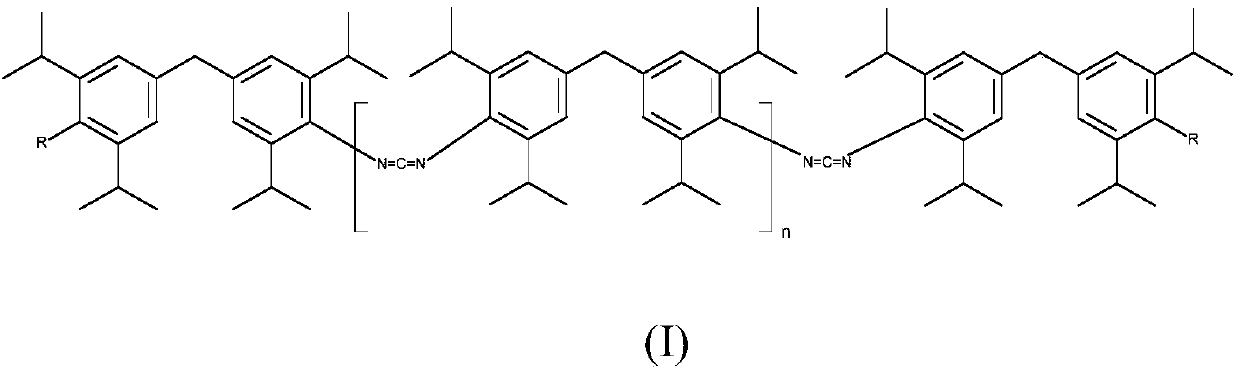
Novel carbodiimides, method for the production and use thereof
The invention relates to novel carbodiimides comprising terminal urea and / or urethane groups of formula (I), in which R can be identical or different and is selected from the group of -NHCONHRI-, -NHCONR'RII- and -NHCOORIII- groups, wherein RI and RII are identical or different and correspond to a CrC-22-alkyl-, C6-C12-cycloalkyl-, C6-C18-aryl- or C7-C18-aralkyl group and RIII corresponds to a C1-C22-alkyl-, C6-C12-cycloalkyl-, C6-C18-aryl- or C7-C18-aralkyl group, or an unsaturated alkyl group with 2-22 carbon atoms, or a alkoxypolyoxyalkylene group, and n = 0 to 20, method for production thereof and use thereof as a stabiliser in ester-based polymers, above all in films for protection against hydrolytic degradation.
Owner:LANXESS DEUTDCHLAND GMBH
Multistage continuous preparation of cycloaliphatic diisocyanates
ActiveUS20050043562A1High yieldImprove availabilityOrganic compound preparationPreparation from ureasPhosgeneDi-isocyanate
Owner:EVONIK OPERATIONS GMBH
Multistage continuous preparation of cycloaliphatic diisocyanates
ActiveUS7329776B2High yieldImprove availabilityOrganic compound preparationPreparation from ureasPhosgeneChemistry
Owner:EVONIK OPERATIONS GMBH
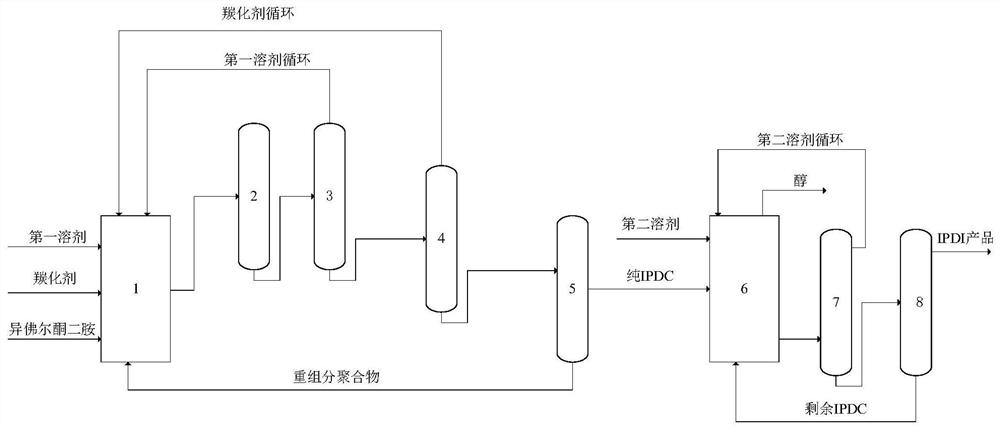
Method for synthesizing isophorone diisocyanate
PendingCN114507161APromote conversionMild reaction conditionsPreparation from ureasPreparation from carbamatesPolymer scienceCarbamate
The invention provides a method for synthesizing isophorone diisocyanate, which comprises the following steps: (1) mixing isophorone diamine, a carbonylation agent, a first solvent and a catalyst, carrying out carbonylation reaction, carrying out solid-liquid separation, and carrying out purification treatment to obtain isophorone dicarbamate; and (2) mixing the isophorone dicarbamate with a second solvent to carry out pyrolytic reaction, and purifying the product to obtain the isophorone diisocyanate. The method is simple in process and free of pollution and potential safety hazards, and the intermediate product isophorone dicarbamate and the final product isophorone diisocyanate are high in yield and purity.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS7307186B2High yieldLow selectivityOrganic compound preparationPreparation from ureasPhosgeneIsocyanate
Owner:DEGUSSA AG
Method for producing tolylene diisocyanate
ActiveUS20140073811A1Carbamic acid derivatives preparationOrganic compound preparationAlcoholToluene
A method for producing tolylene diisocyanate includes: mixing a first diaminotoluene containing 2,4-diaminotoluene and 2,6-diaminotoluene at a first isomer ratio and a second diaminotoluene containing 2,4-diaminotoluene and / or 2,6-diaminotoluene at a second isomer ratio that is different from the first isomer ratio so as to prepare mixed diaminotoluene; producing tolylene dicarbamate by reaction of the mixed diaminotoluene, urea and / or N-unsubstituted carbamic acid ester and alcohol; and thermally decomposing the tolylene dicarbamate.
Owner:MITSUI CHEM INC
Low chlorine, multi-staged method for producing cycloaliphatic disocyanates
InactiveUS8536370B2Reduce environmental pollutionSuppress formation of N-methylPreparation from carboxylic acid nitrogen analoguesOrganic compound preparationAlkaneAlcohol
Low chlorine, multi-staged method for producing cycloaliphatic diisocyanates. The invention relates to a multi-staged method for the continuous low-chlorine production of cycloaliphatic diisocyanates, comprising the synthesis of diaminodipheynl alkanes, the hydration thereof into the corresponding cycloaliphatic diamines and the subsequent conversion of cycloaliphatic diamines to the corresponding cycloalkylene biscarbamates and the thermal cleaving of the latter into the cycloaliphatic diisocyanates and alcohol.
Owner:EVONIK DEGUSSA GMBH
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS7339074B2Speed up the processOrganic compound preparationPreparation from ureasPhosgeneChemistry
The invention relates to a multistage process for continuous and phosgene-free preparation of cycloaliphatic diisocyanates.
Owner:DEGUSSA AG
Method for manufacturing tolylene diisocyanate
A method for manufacturing tolylene diisocyanate comprises a mixing step, a carbamate manufacturing step, and a pyrolysis step. In the mixing step, a first diaminotoluene containing 2,4- and 2,6-diaminotoluene in a first isomeric ratio is mixed with a second diaminotoluene containing 2,4- and / or 2,6-diaminotoluene in a second isomeric ratio, and mixed diaminotoluene is prepared. In the carbamate manufacturing step, a reaction is brought about between the mixed diaminotoluene prepared in the mixing step, urea and / or N-unsubstituted carbamate, and an alcohol, whereby tolylene dicarbamate is produced. In the pyrolysis step, the tolylene dicarbamate is pyrolyzed.
Owner:MITSUI CHEM INC
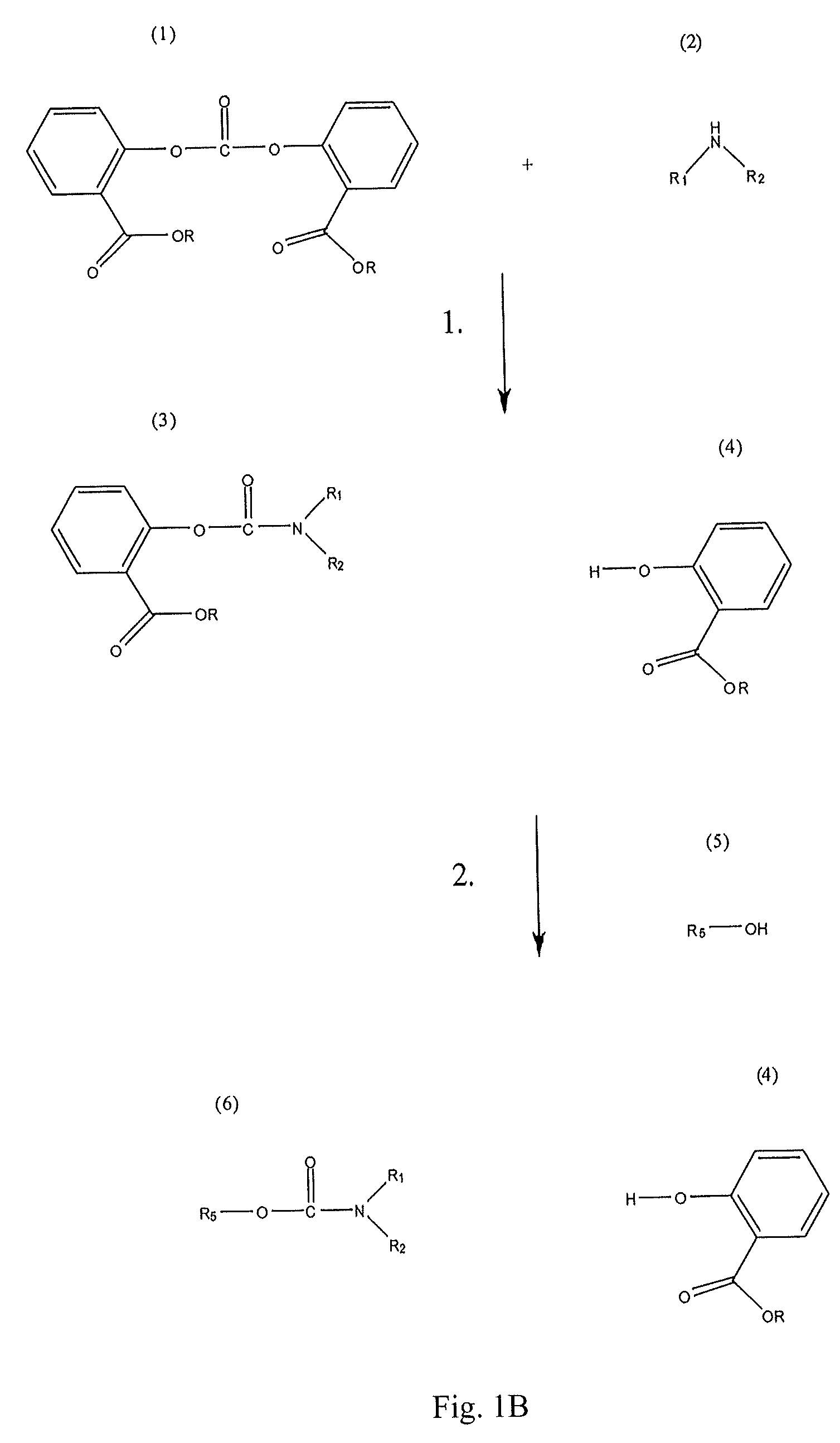
Method for making carbamates, ureas and isocyanates
InactiveUS8058469B2High product yieldShort timeUrea derivatives preparationCarbamic acid derivatives preparationCarbamatePyrolysis
Owner:SABIC GLOBAL TECH BV
Preparation method of alkyl substitution diphenylmethane diisocyanate
InactiveCN102516125AHigh yieldImprove yieldPreparation from ureasPreparation from carbamatesDiphenylmethaneSodium methoxide
The invention discloses a preparation method of alkyl substitution diphenylmethane diisocyanate, which includes the following steps: weighing alkylaniline and urea, placing the alkylaniline and the urea in a reactor, heating the alkylaniline and the urea to the temperature ranging from 100 DEG C to 200 DEG C, stirring the mixture for 10 to 20 minutes, stopping reaction when red liquid is changed to solid, cooling the mixture to the room temperature, washing the mixture for 3 to 6 times through alcohol to obtain dialkyl group diphenyl urea, adding dimethyl carbonate into dialkyl group diphenyl urea, conducting back flowing reaction on the mixture under the temperature ranging from 110 DEG C to 250 DEG C for 6 to 12 hours, adding sodium methoxide into the mixture, conducting filtering after reaction is finished, conducting reduced pressure distillation on filtering liquid, conducting crystallization on normal hexane to obtain alkyl aniline methyl formate, adding lewis acid and methylation agent into the alkyl aniline methyl formate, increasing the temperature to the range from 70 DEG C to 100 DEG C, conducting reaction for 4 to 7 hours, cooling the mixture to the room temperature, conducting filtering on the mixture and washing the mixture through alcohol for 3 to 6 times to obtain alkyl aniline methyl formate and conducting decomposition reaction on the dialkyl group diphenylmethane dicarbamic acid ester. The preparation method of the alkyl substitution diphenylmethane diisocyanate is strong in stability, low in toxicity and controllable in reaction process.
Owner:GUANGZHOU SUPER CHEM COATING CO LTDGUANGZHOU SUPER CHEM COATING CO LTD
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS7420080B2Speed up the processImprove availabilityPreparation from carboxylic acid nitrogen analoguesOrganic compound preparationPolymer sciencePhosgene
The invention relates to a multistage process for continuous and phosgene-free preparation of cycloaliphatic diisocyanates.
Owner:EVONIK DEGUSSA GMBH
Multistage continuous preparation of cycloaliphatic disocyanates
Cycloaliphatic diisocyanates can be prepared continuously and phosgene-free by reacting at least one cycloaliphatic diamine with at least one carbonic acid derivative and at least one alcohol to give a cycloaliphatic diurethane; freeing the cycloaliphatic diurethane of a low boiler, a medium boiler and mixtures thereof; thermally cleaving the cycloaliphatic diurethane in a cleavage apparatus to give a cycloaliphatic diisocyanate and a cleavage residue; continuously discharging a portion of the cleavage residue from the cleavage apparatus; removing the high boiler components from the discharge to obtain a purified discharge; reurethanizing the purified discharge with alcohol to obtain a reurethanized discharge; and recycling the reurethanized discharge into the process.
Owner:EVONIK DEGUSSA GMBH
Multi-step method for continuously producing toluene diisocyanate
The present invention provides a multi-step method for continuously producing alicyclic vulcabond on the condition of non light and non-air, by the reaction of the alicyclic diamine and the carboxylic acid derivatives with alcohol producing alicyclic diurethane and subsequently decomposed into alicyclic vulcabond, characterized in that diurethane is formed by two steps, thermo-decomposing via removing diurethane of the easier boiler and the medium boiler to release desirable vulcabond, and continuously draining the product on the bottom of the part degradable tower of the degradable equipment, proceeding re-urethane by alcohol, then separating the high boiler, and then making the re-urethane product circle in the process, wherein using the unprocessed carbamide and / or carbamide equivalent made of un-precessed carbamide.
Owner:GOLDSCHMIDT CHEM CORP
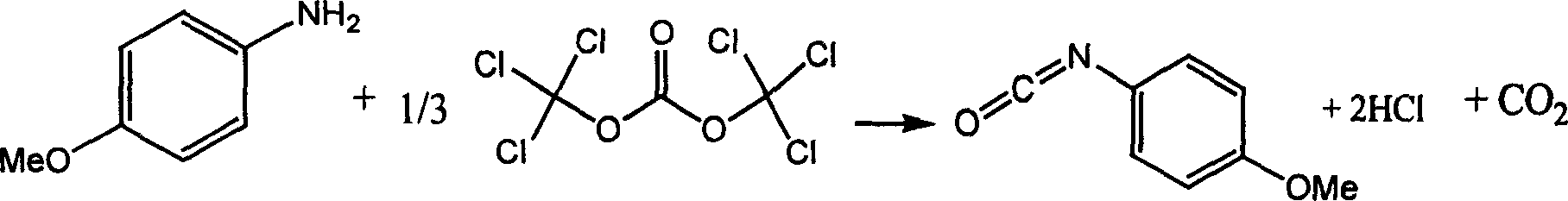
Chemical synthesis of p-methoxyphenyl isocyanate
InactiveCN1468845AAdvanced process routeReasonable process conditionsPreparation from ureasChemical synthesisChlorobenzene
The chemical synthesis of p-methoxyphenyl isocyanate is one process inside organic solvent with methoxyphenyl amine and di(trichloromethyl) carbonate as material and in the presence of catalyst. The process has the material molar ratio of methoxyphenyl amine to di(trichloromethyl) carbonate to catalyst being 1 to 0.34-0.8 to 0.01-0.1; organic solvent amount is 3-12 times mass of methoxyphenyl amine; the reaction temperature is 20-180 deg.c and reaction period is 4-10 hr. The organic solvent may be benzene, toluene, xylene, chlorobenzene, dichloro benzene, dichloro methane, trichloro methane, carbon tetrachloride, ethylidene dichloride, tetrafuran or hexaalkane epoxide. The said process uses no virulent phosgene and diphosgene, and is safe, reliable, high in yield, low in cost, and less corrosive and waste exhaust.
Owner:ZHEJIANG TONGFENG PHARMA & CHEM
A kind of urea method ionic liquid catalysis prepares the method for toluene diisocyanate
ActiveCN104276982BImprove catalytic performanceEasy to removePreparation from ureasOrganic-compounds/hydrides/coordination-complexes catalystsToluene diisocyanateDevice material
The invention provides application of an ionic liquid in preparing toluene diisocynate and a method for preparing the toluene diisocynate from urea under the catalysis of the ionic liquid. The method is used for preparing the toluene diisocynate by taking toluenediamine and urea as the raw materials, reacting the raw materials to generate toluene diurea under the catalytic action of the ionic liquid and then pyrolyzing the toluene diurea. The method does not need highly toxic phosgene and an extra solvent; the ionic liquid is adopted as a catalyst; on one hand, the ionic liquid is good in catalytic effect, easy to remove and environment-friendly, and on the other hand, a metal catalyst in the prior art is not used, so that the reaction of the method for preparing the toluene diisocynate from urea under the catalysis of the ionic liquid does not require harsh device materials and operating conditions, consequently, the production cost is greatly reduced, and the method is advantageous to industrial application.
Owner:陕西煤业化工技术开发中心有限责任公司
Multistage continuous preparation of cycloaliphatic diisocyanates
InactiveUS20050267310A1Speed up the processAvoid disadvantagesPreparation from carboxylic acid nitrogen analoguesOrganic compound preparationPhosgeneDi-isocyanate
The invention relates to a multistage process for continuous and phosgene-free preparation of cycloaliphatic diisocyanates.
Owner:EVONIK DEGUSSA GMBH
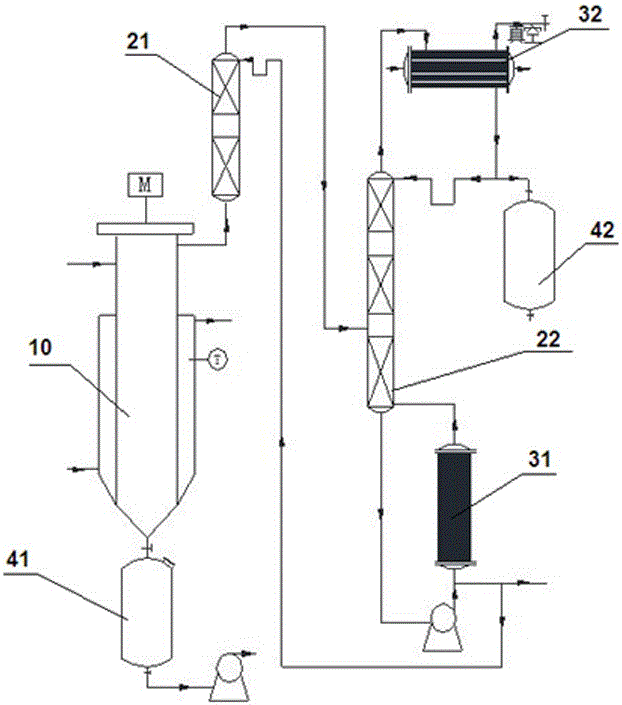
System integrating fatty group or ring group diisocyanate synthesis and separation and purification and synthesis method
InactiveCN105801450AHigh purityEfficient separationPreparation from ureasChemical industrySynthesis methodsTower
The invention discloses a system integrating fatty group or ring group diisocyanate synthesis and separation and purification and a synthesis method. The system comprises a wiped film vaporizer, a first rectifying tower, a second rectifying tower, a first heat exchanger and a second heat exchanger; the wiped film vaporizer is communicated with the first rectifying tower through a pipe, an outlet pipe of the first rectifying tower is communicated with the middle of the second rectifying tower, the bottom of the second rectifying tower is communicated with the first heat exchanger, and the top of the second rectifying tower is communicated with the second heat exchanger. By means of the system integrating fatty group or ring group diisocyanate synthesis and separation and purification, components generated by pyrolysis can be separated efficiently, and the system is quite suitable for a heat sensitivity material system; by the adoption of the system, materials which do not react completely are returned to a reactor for continuous pyrolysis, a diisocyanate product and light components are subjected to extraction with the rectifying towers, a coarse product is high in purity, energy is saved, consumption is reduced, and the diisocyanate product is good in selectivity and high in yield.
Owner:ANHUI HUARONG GAOKE NEW MATERIAL CO LTD
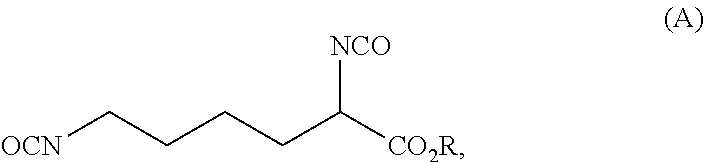
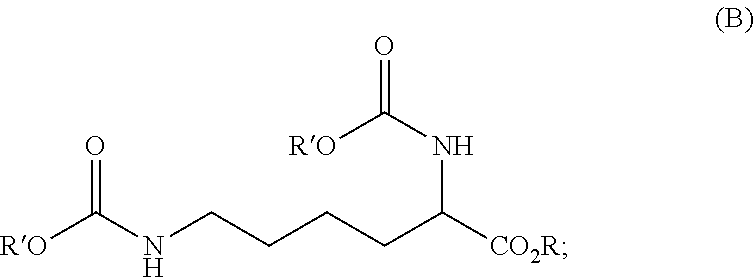
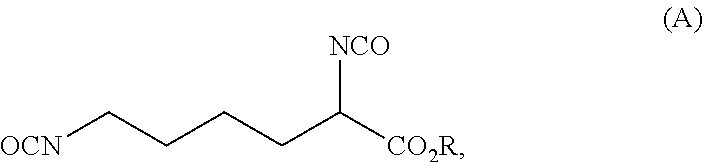
Process for preparing diisocyanates based on lysine
ActiveUS20210403417A1Reduce health risksCost efficientUrea derivatives preparationCarbamic acid derivatives preparationArylPolymer science
The invention relates to a process for preparing a diisocyanate of the formula (A)where R is selected from the group consisting of alkyl, aryl, and combinations thereof, comprising the following process steps in the indicated order;1) providing an intermediate of the formula (B) with a process using lysine and ureaand where R and each R′ are independently selected from the group consisting of alkyl, aryl, and combinations thereof; and2) thermolytic cleavage of the intermediate of the formula (B),thereby affording the diisocyanate of the formula (A),and also to the diisocyanate directly prepared therewith.
Owner:EVONIK OPERATIONS GMBH
Process for producing bromo chloro isocyanuric acid and its use
This invention relates to a manufacturing technique and its use of chlorine bromine isocyanuric acid. Urea and cyanamide are cyclized to cyanuric acid, and then be made into cyanuric acid suspension. Sodium hypochlorite and sodium hypobromite are added to make chlorination and bromination reaction. Chlorine bromine isocyanuric acid is got after filtration, scrubbing to filter cake, srying of the resultant. The compound of chlorine bromine isocyanuric acid and light fertilizer can be used to produce agriculture chemicals fungicide. The sodium hypochlorite and sodium hypobromite are added with no order, so its technique is convenient to control. Comparing to exist technique using chlorine and bromine gas, the sodium hypochlorite and sodium hypobromite used are liquor and hard to evaporate. The equipment request is not high, and the equipment cost can be reduced greatly, mass production cost also can be greatly reduced.
Owner:JIANGSU DONGBAO AGROCHEM
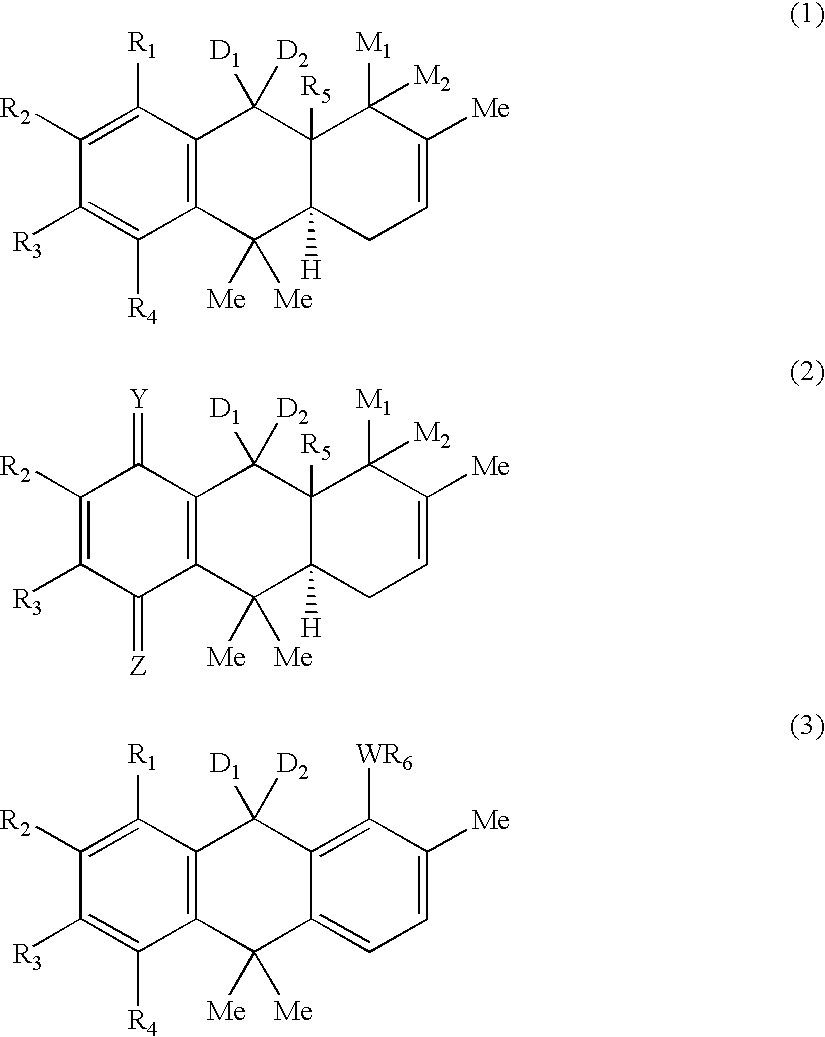
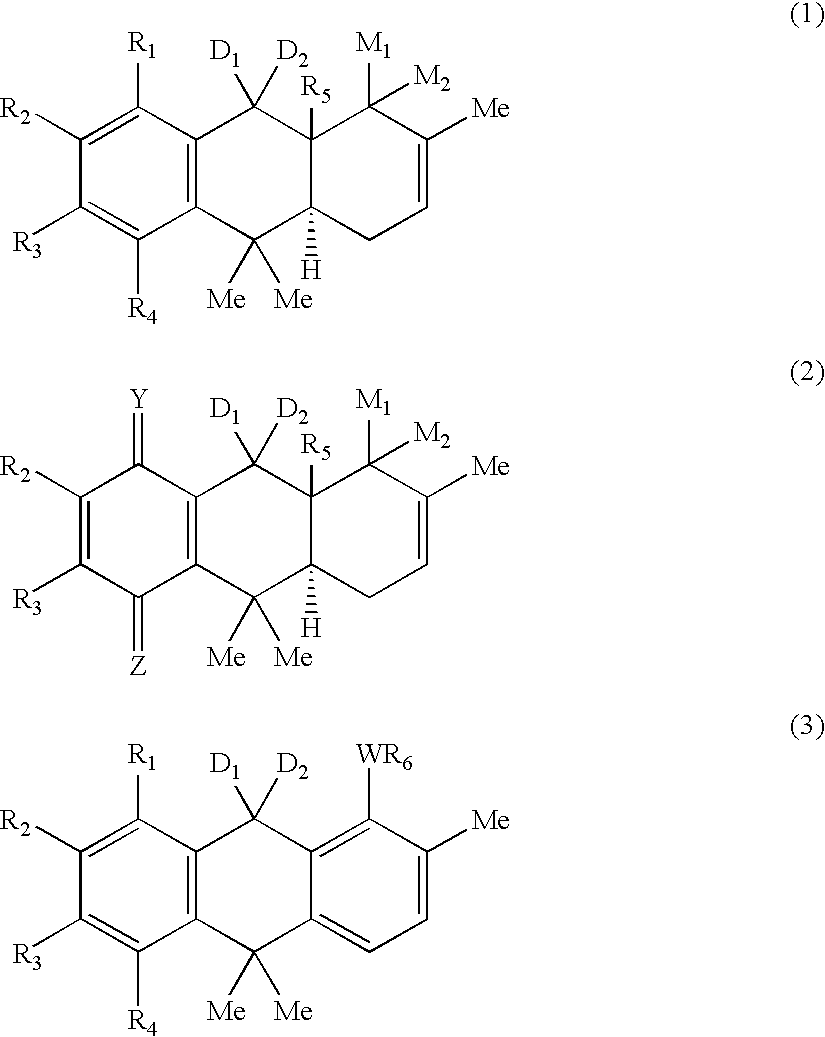
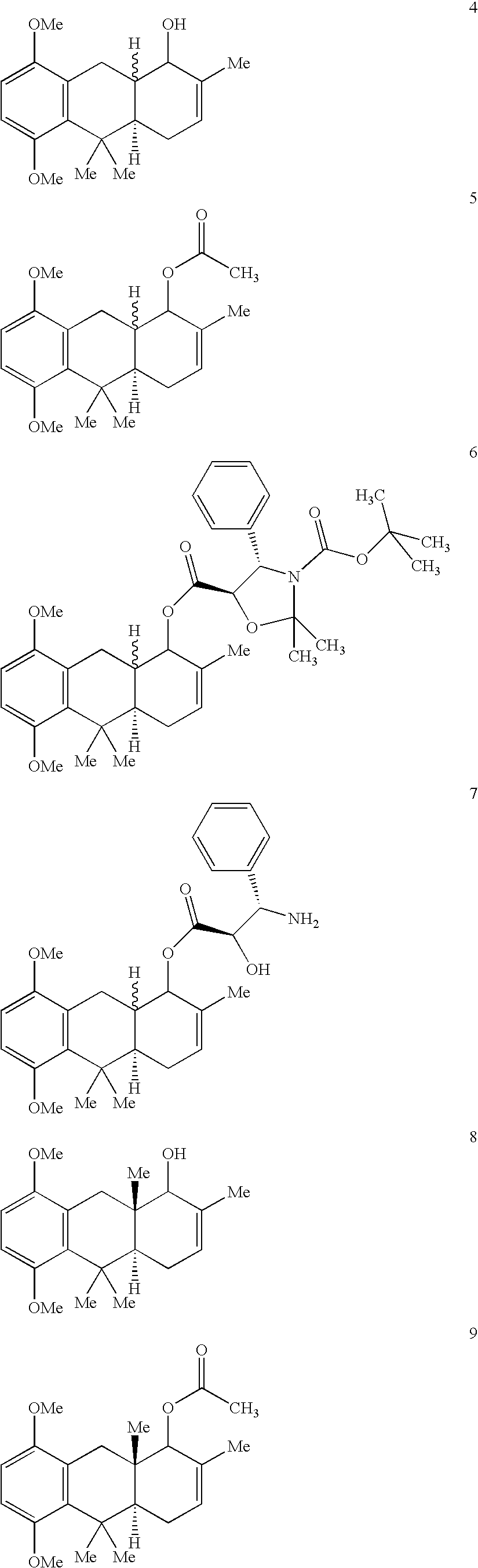
Hydroanthracene based compounds as anticancer agents
The invention relates to the use of novel anthracene based compounds for the inhibition or prevention of the growth or multiplication of cancer cells, and to therapeutic compositions containing such compounds. The invention relates more specifically to the use of hydroanthracene based compounds for the inhibition and / or prevention of cancer of the colon, pancreas, prostate, lung, larynx, ovary, breast, glioblastoma, oral cavity, endothelial cells and leukemias. The agents of the invention form a distinct class, distinct from the anthracene, anthrone or anthraquinone derivatives.
Owner:DABUR PHARM LTD
Carbodiimides, method for the production and use thereof
ActiveUS10065925B2Improve thermal stabilityReduce exhaustPreparation from ureasCarbamateStabilizing Agents
The invention relates to novel carbodiimides having terminal urea and / or urethane groups, to processes for the production thereof and to the use thereof as a stabilizer in ester-based polymers especially in films for protection from hydrolytic degradation.
Owner:LANXESS DEUTDCHLAND GMBH
Method for producing tolylene diisocyanate
A method for producing tolylene diisocyanate includes: mixing a first diaminotoluene containing 2,4-diaminotoluene and 2,6-diaminotoluene at a first isomer ratio and a second diaminotoluene containing 2,4-diaminotoluene and / or 2,6-diaminotoluene at a second isomer ratio that is different from the first isomer ratio so as to prepare mixed diaminotoluene; producing tolylene dicarbamate by reaction of the mixed diaminotoluene, urea and / or N-unsubstituted carbamic acid ester and alcohol; and thermally decomposing the tolylene dicarbamate.
Owner:MITSUI CHEM INC
Novel carbodiimides, processes for their production and uses
The present invention relates to novel carbodiimides comprising terminal urea and / or urethane groups having the formula (I), wherein R may be the same or different and are selected from -NHCONHR I ‑,‑NHCONR'R II ‑and‑NHCOOR III ‑groups of groups in which R I and R II are the same or different and correspond to CrC-22-alkyl-, C 6 -C 12 ‑Cycloalkyl‑, C 6 -C 18 ‑aryl‑ or C 7 -C 18 ‑aralkyl group, and R III corresponds to C 1 -C 22 ‑Alkyl‑, C 6 -C 12 ‑Cycloalkyl‑, C 6 -C 18 ‑aryl‑ or C 7 -C 18 ‑aralkyl groups, or unsaturated alkyl groups having 2‑22 carbon atoms, or alkoxypolyoxyalkylene groups, and n=0 to 20; their production and their use as stabilizers in esters Use of polymeroids, especially in membranes for protection against hydrolytic degradation.
Owner:LANXESS DEUTDCHLAND GMBH
Chemical synthesis of p-methoxyphenyl isocyanate
InactiveCN1228316CAdvanced process routeReasonable process conditionsPreparation from ureasChemical synthesisChlorobenzene
The chemical synthesis of p-methoxyphenyl isocyanate is one process inside organic solvent with methoxyphenyl amine and di(trichloromethyl) carbonate as material and in the presence of catalyst. The process has the material molar ratio of methoxyphenyl amine to di(trichloromethyl) carbonate to catalyst being 1 to 0.34-0.8 to 0.01-0.1; organic solvent amount is 3-12 times mass of methoxyphenyl amine; the reaction temperature is 20-180 deg.c and reaction period is 4-10 hr. The organic solvent may be benzene, toluene, xylene, chlorobenzene, dichloro benzene, dichloro methane, trichloro methane, carbon tetrachloride, ethylidene dichloride, tetrafuran or hexaalkane epoxide. The said process uses no virulent phosgene and diphosgene, and is safe, reliable, high in yield, low in cost, and less corrosive and waste exhaust.
Owner:ZHEJIANG TONGFENG PHARMA & CHEM
Method for preparing toluene diisocynate from urea under catalysis of ionic liquid
ActiveCN104276982AImprove catalytic performanceEasy to removePreparation from ureasOrganic-compounds/hydrides/coordination-complexes catalystsDevice materialCatalytic effect
The invention provides application of an ionic liquid in preparing toluene diisocynate and a method for preparing the toluene diisocynate from urea under the catalysis of the ionic liquid. The method is used for preparing the toluene diisocynate by taking toluenediamine and urea as the raw materials, reacting the raw materials to generate toluene diurea under the catalytic action of the ionic liquid and then pyrolyzing the toluene diurea. The method does not need highly toxic phosgene and an extra solvent; the ionic liquid is adopted as a catalyst; on one hand, the ionic liquid is good in catalytic effect, easy to remove and environment-friendly, and on the other hand, a metal catalyst in the prior art is not used, so that the reaction of the method for preparing the toluene diisocynate from urea under the catalysis of the ionic liquid does not require harsh device materials and operating conditions, consequently, the production cost is greatly reduced, and the method is advantageous to industrial application.
Owner:陕西煤业化工技术开发中心有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com