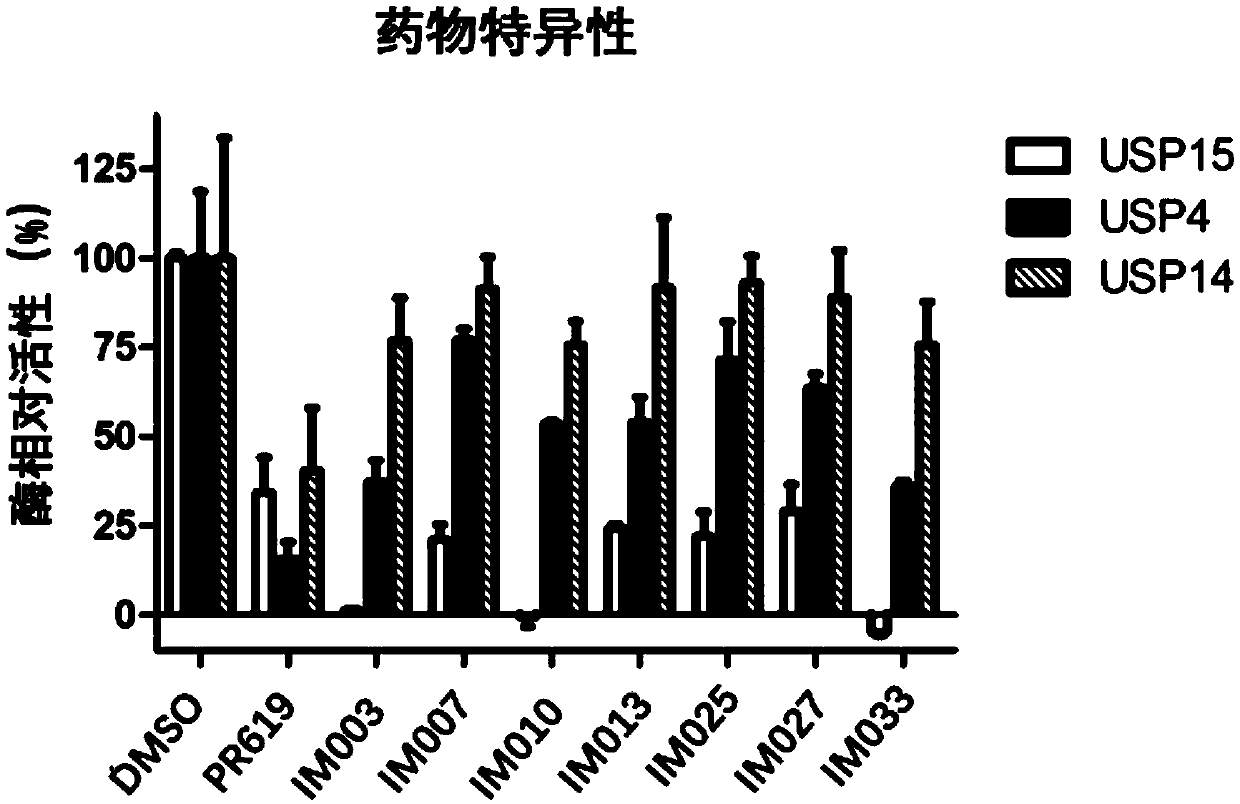
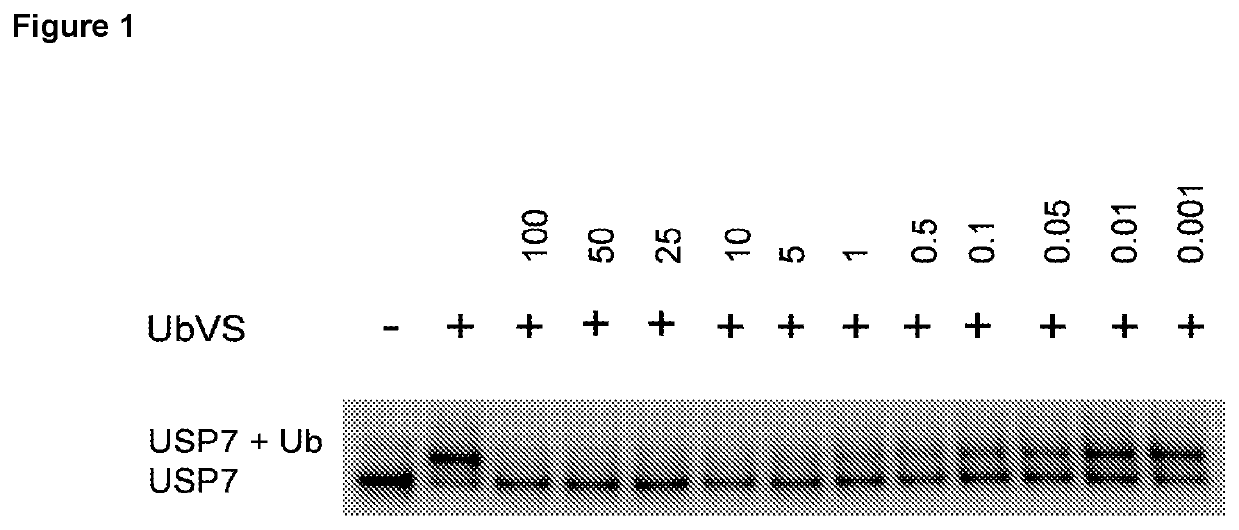
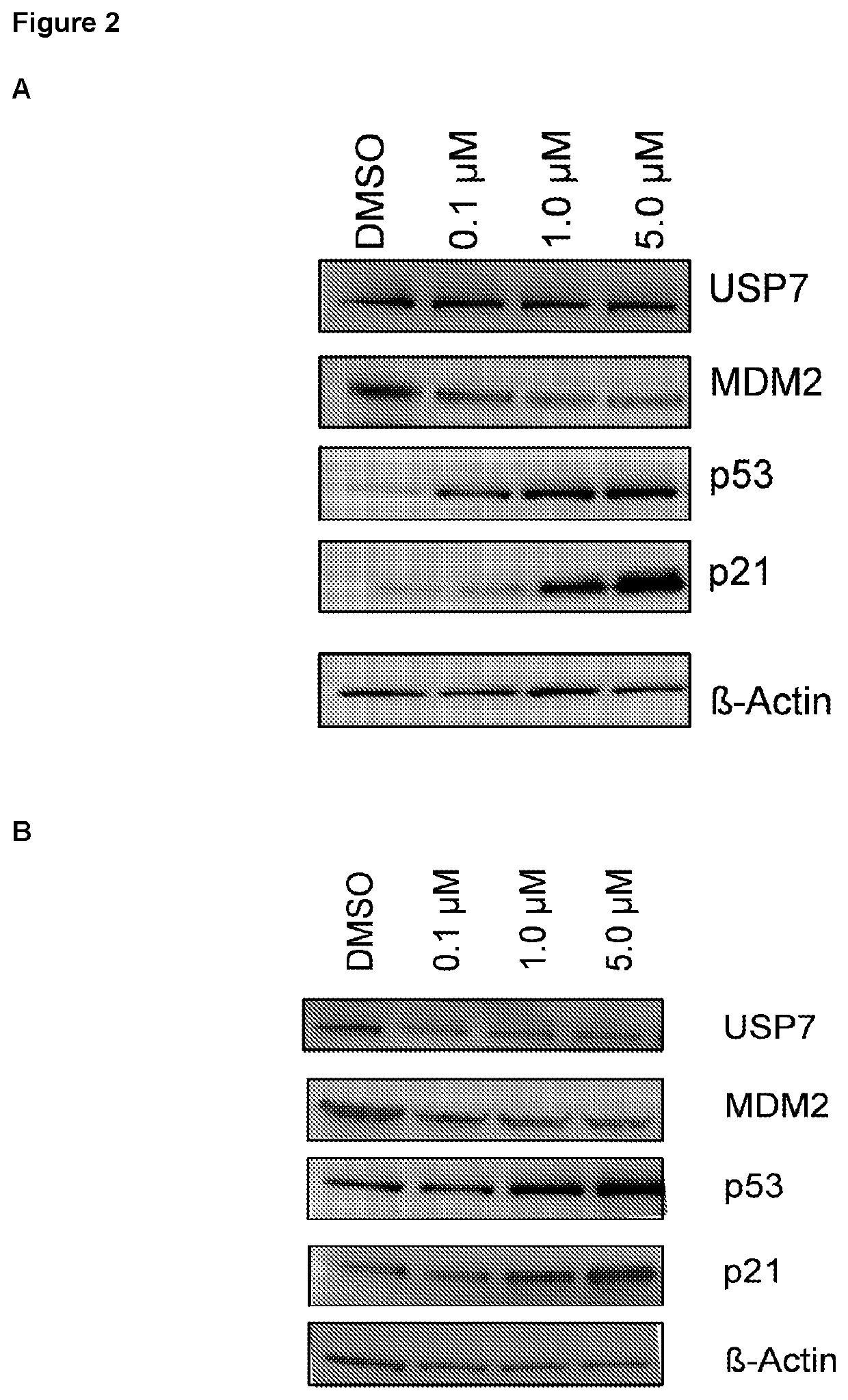
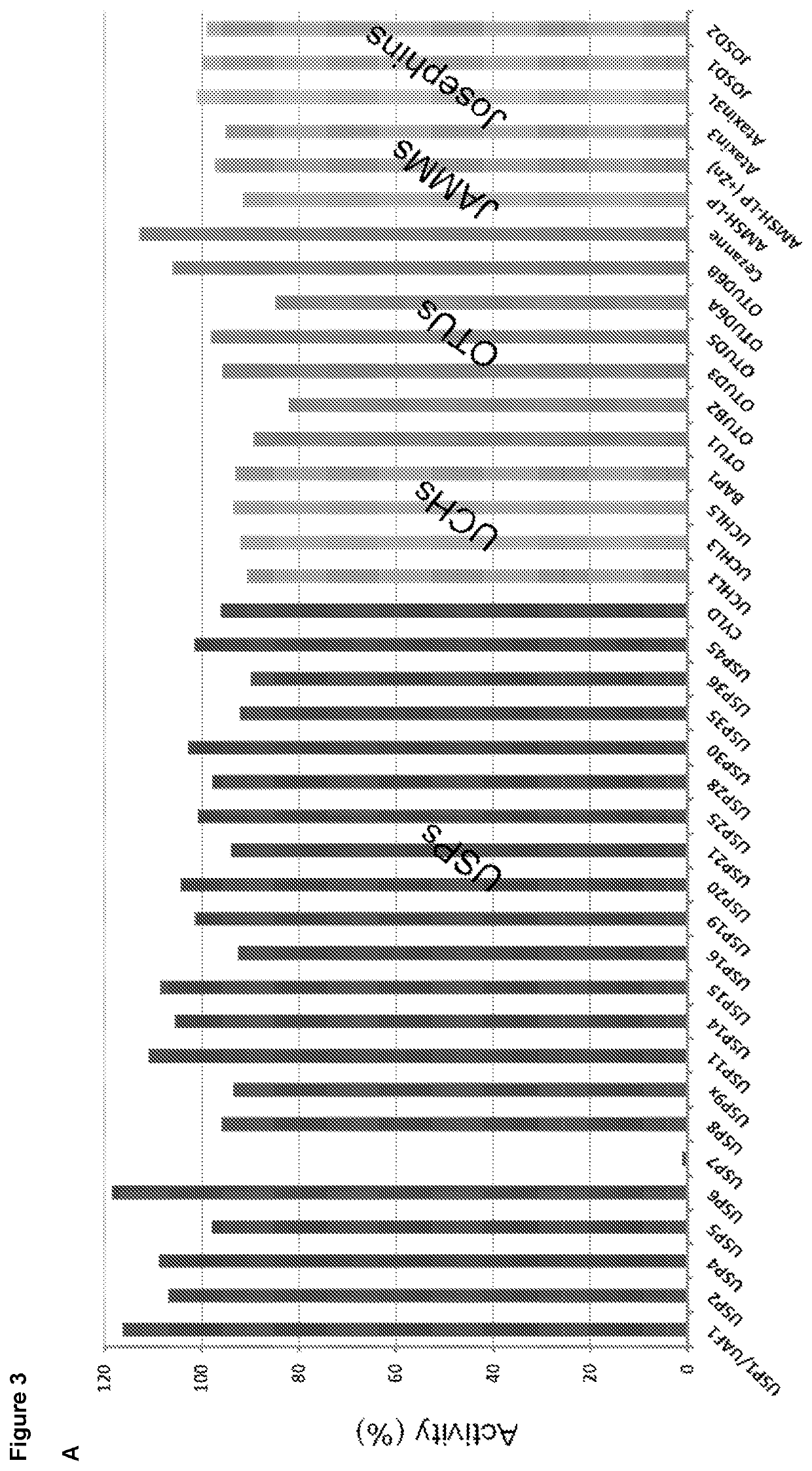
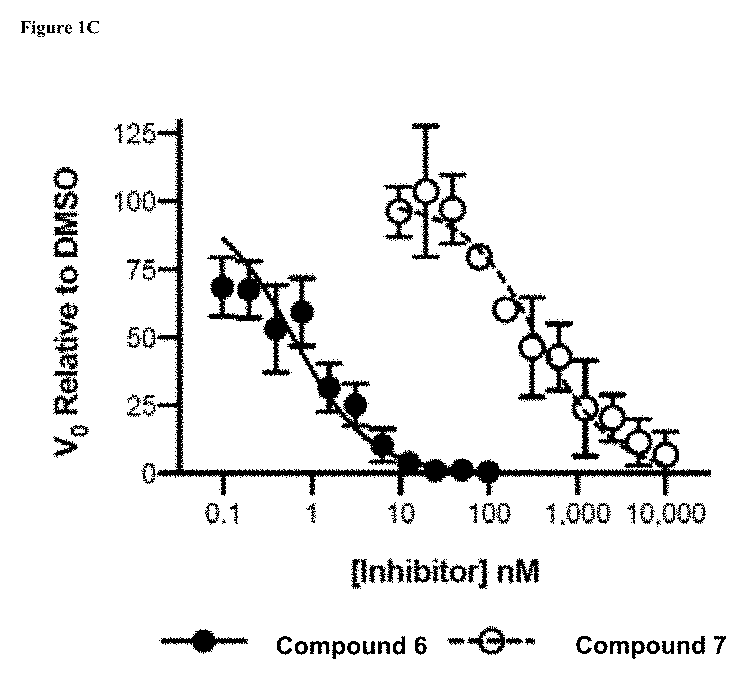
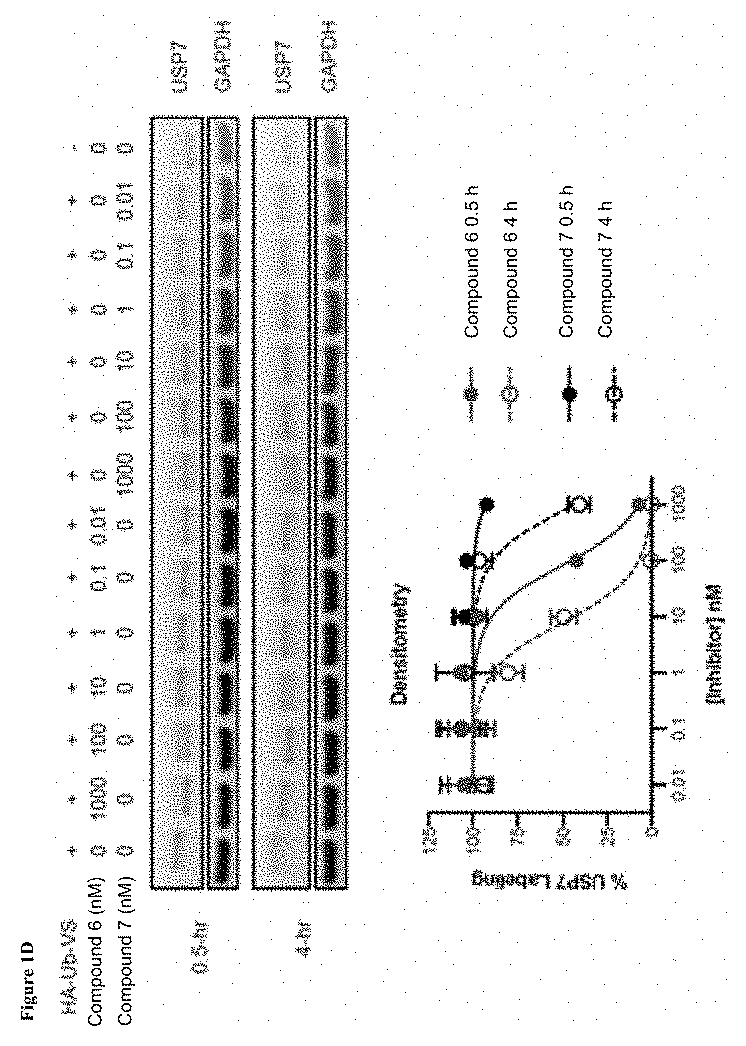
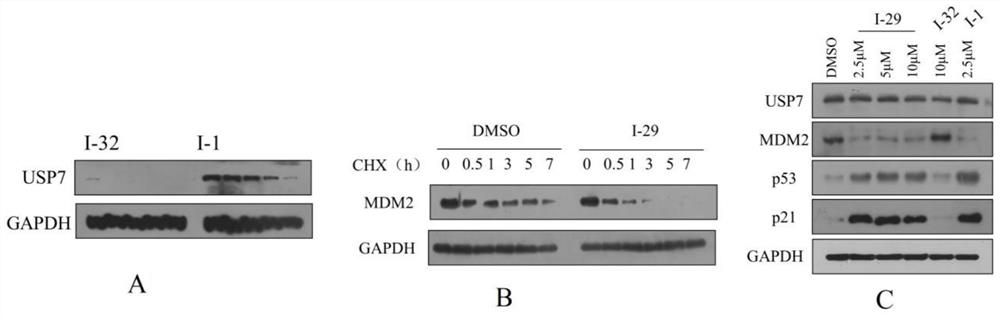
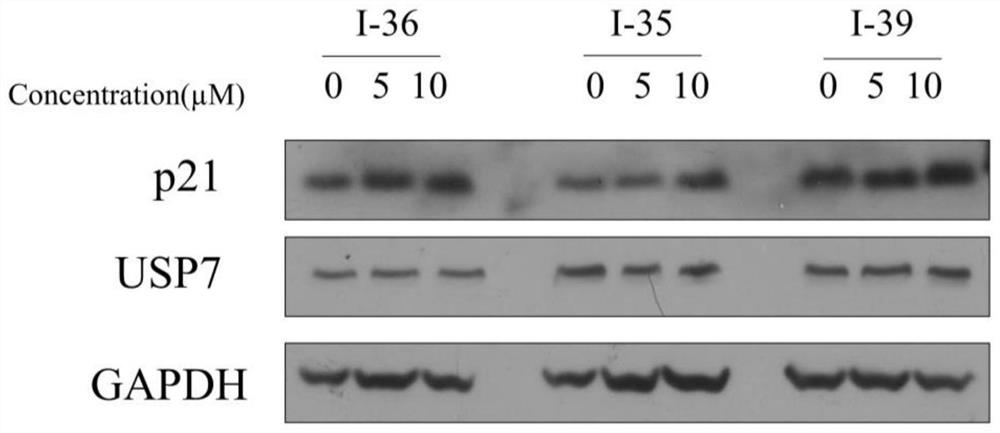
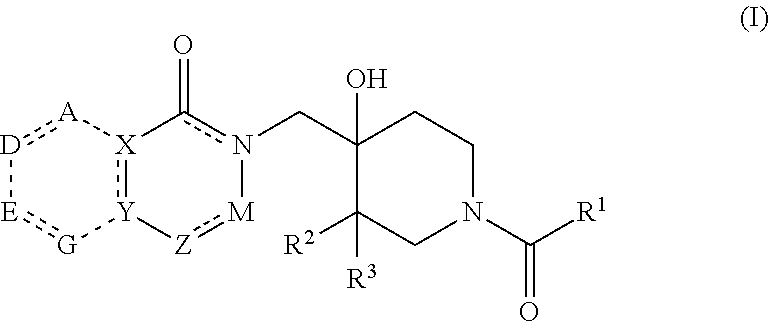
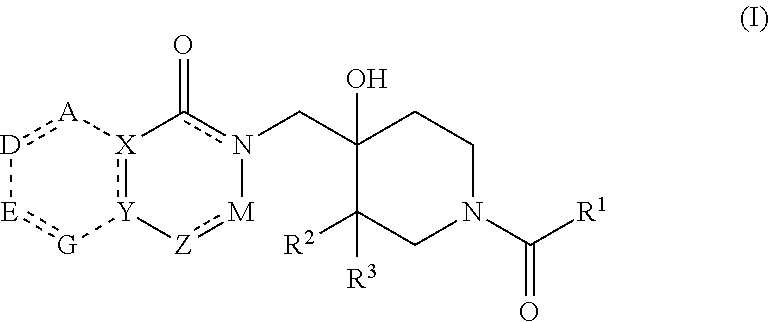
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Ubiquitin specific protease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
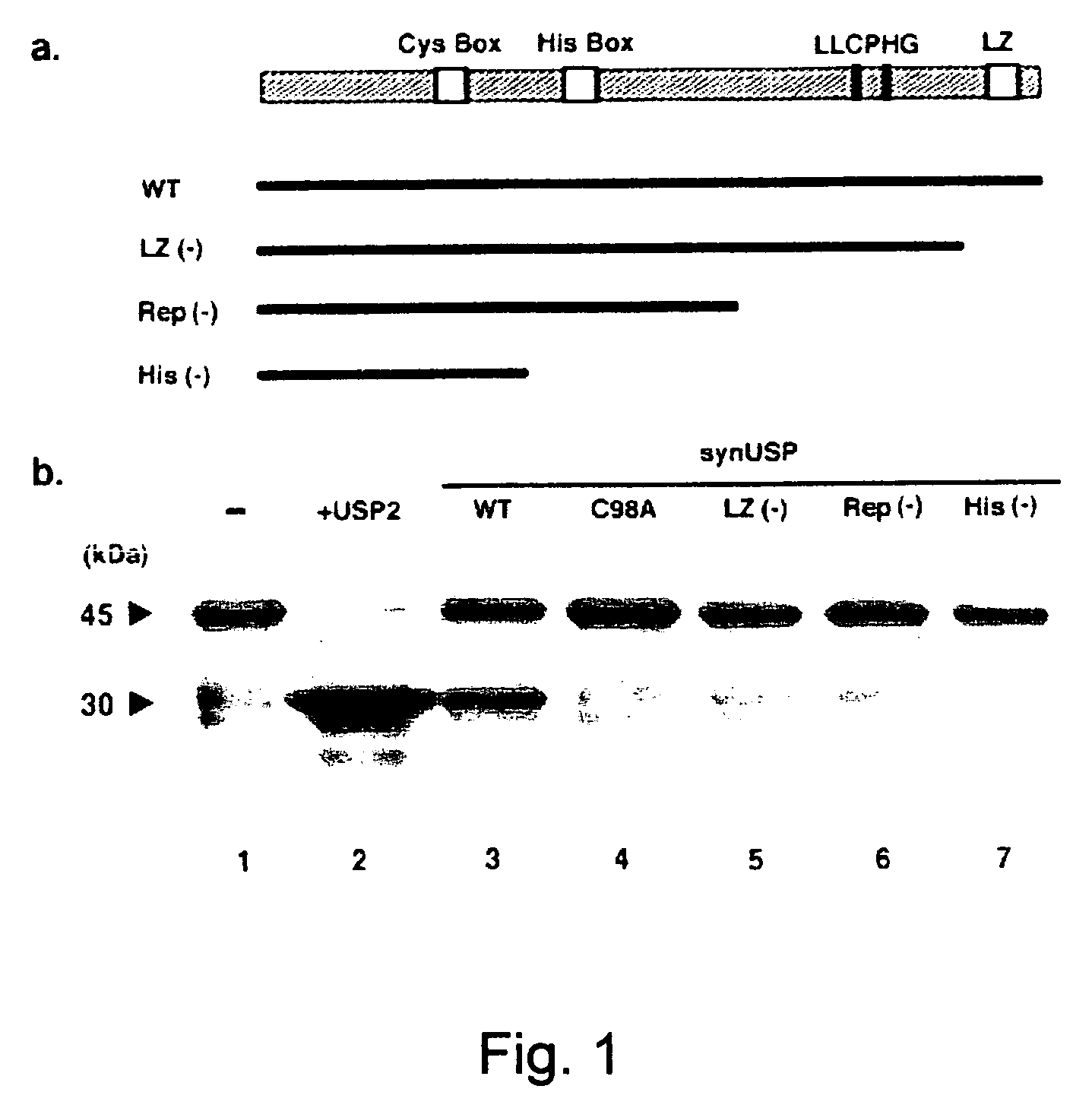
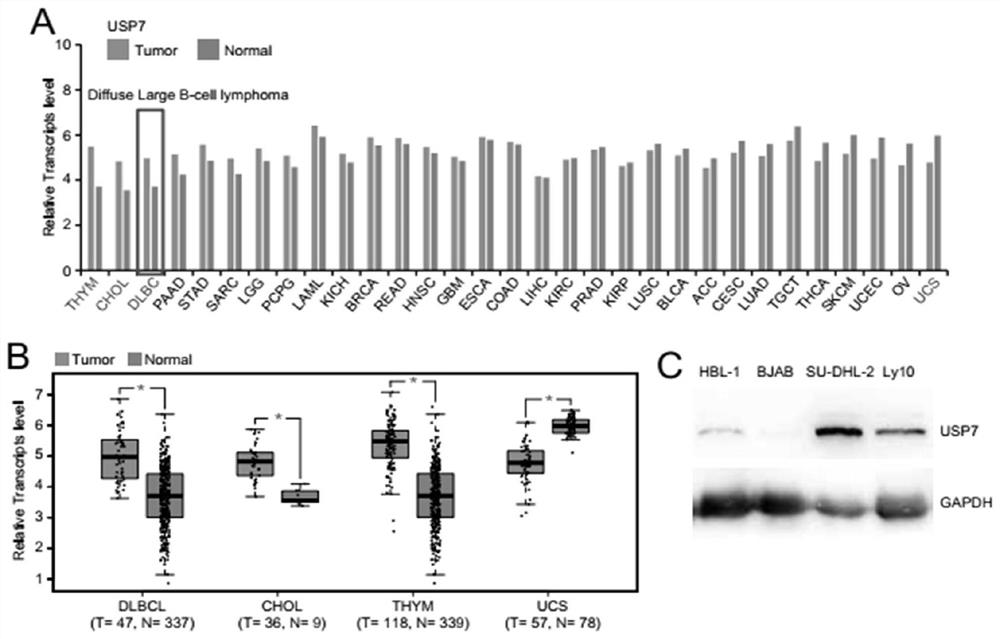
Ubiquitin specific protease 7 (USP7) is a deubiquitinase, an enzyme that removes ubiquitin a 76 amino acid protein that is added onto lysines in the target protein. Proteins that are mono, or poly (up to 10 residues), ubiquitinated are taken to the proteasome for destruction.
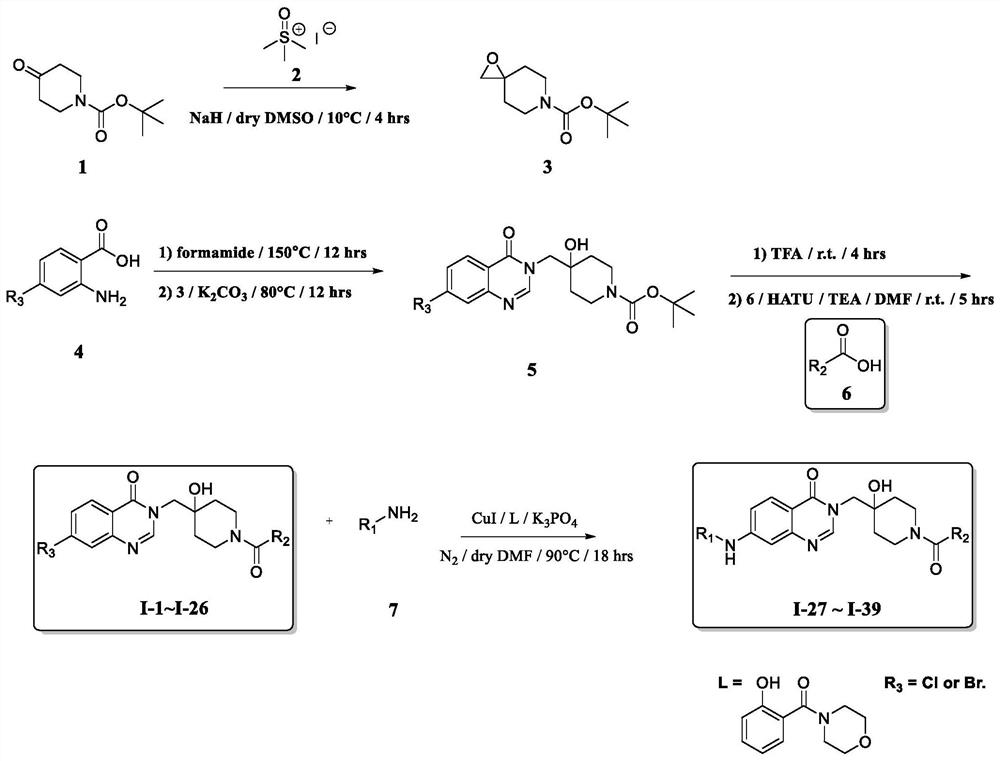
Substituted quinazolin-4-ones for inhibiting ubiquitin specific protease 7
ActiveUS9546150B2Easy to identifyOrganic active ingredientsNervous disorderKetoneNeuro-degenerative disease
The present invention relates to quinazolin-4-one compounds of formula (I′), their process of preparation and uses thereof. These compounds are useful as selective and reversible inhibitors of ubiquitin specific proteases, particularly USP7, for treating e.g. cancer, neurodegenerative diseases, inflammatory disorders and viral infections.
Owner:HYBRIGENICS SA
Ubiquitin-specific protease
The present invention provides novel members of the family of deubiquitinating enzymes (DUBs) as well as biologically, diagnostically or therapeutically useful fragments, derivatives, and homologues thereof, which are useful in research, biological, clinical, therapeutic as well as prognostic and diagnostic purposes. Furthermore, the polynucleotides and polypeptides provided by the present invention are useful for the treatment and diagnosis of breast cancer, leukemia or brain disorders.
Owner:NOVARTIS AG
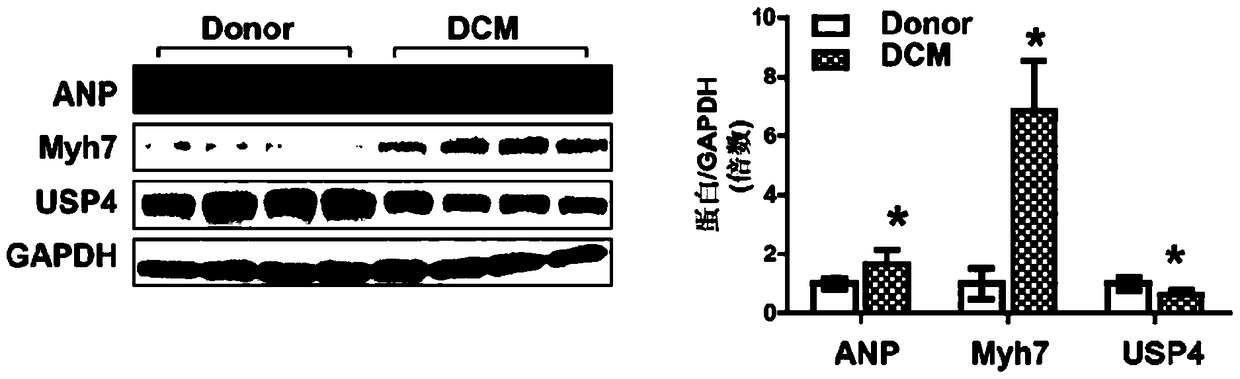
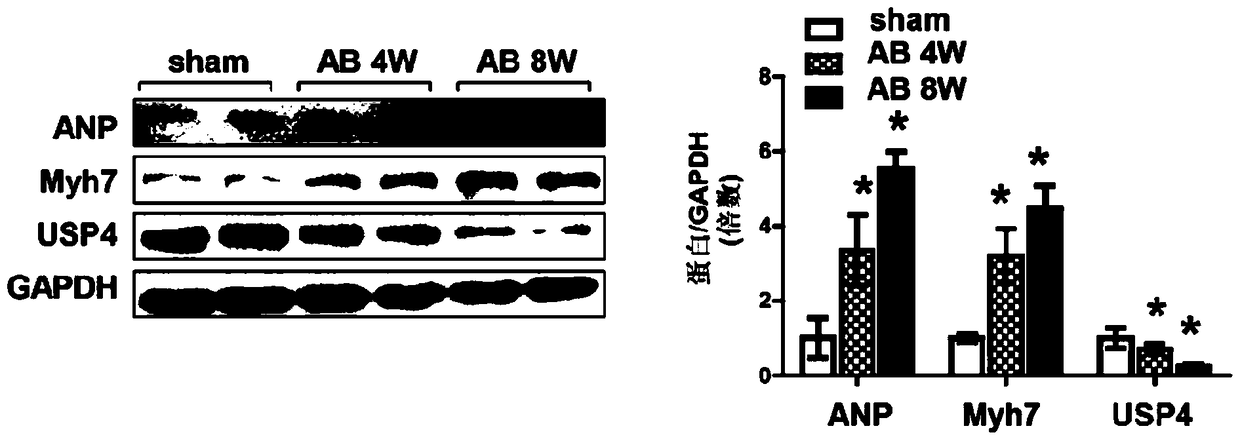
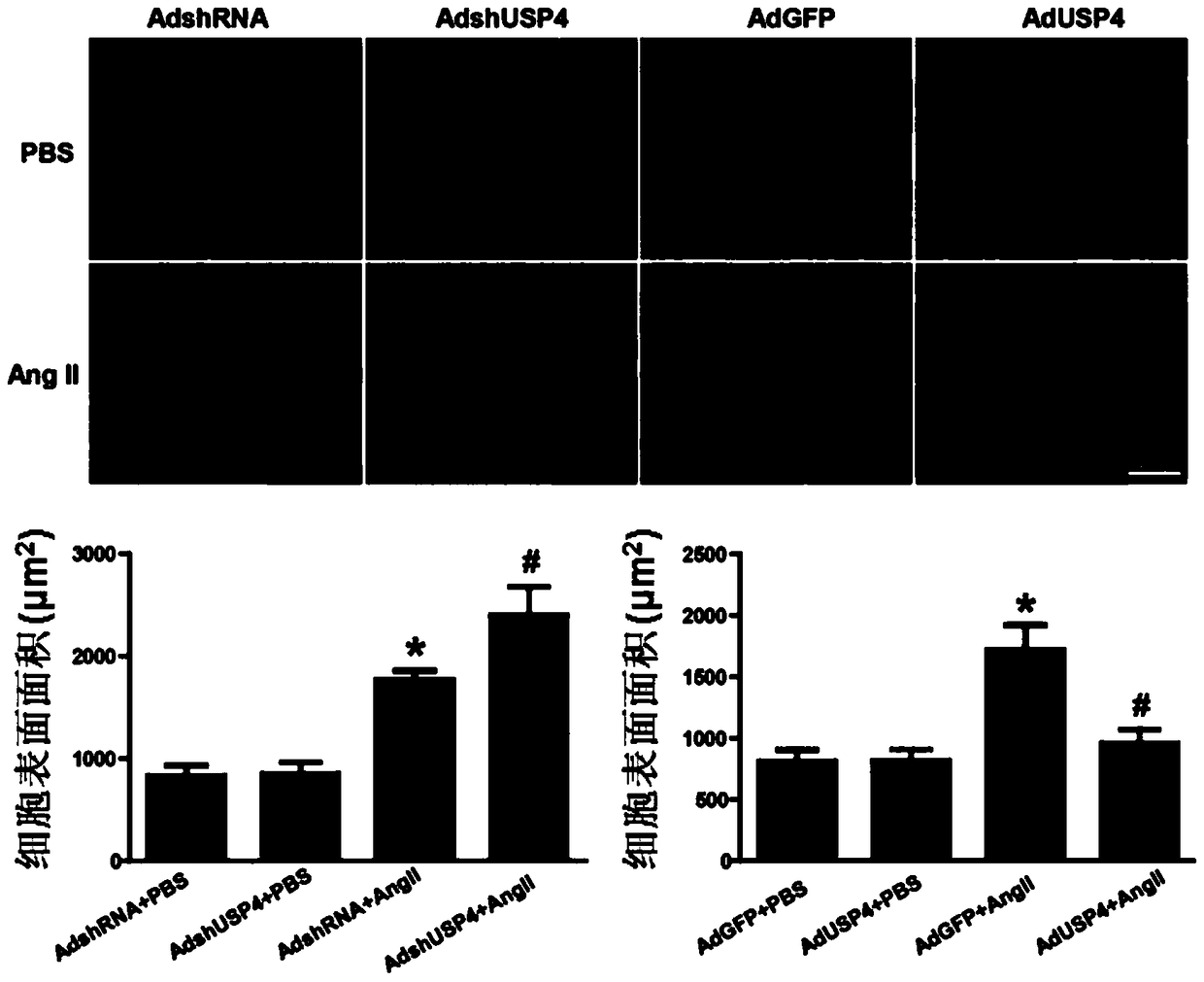
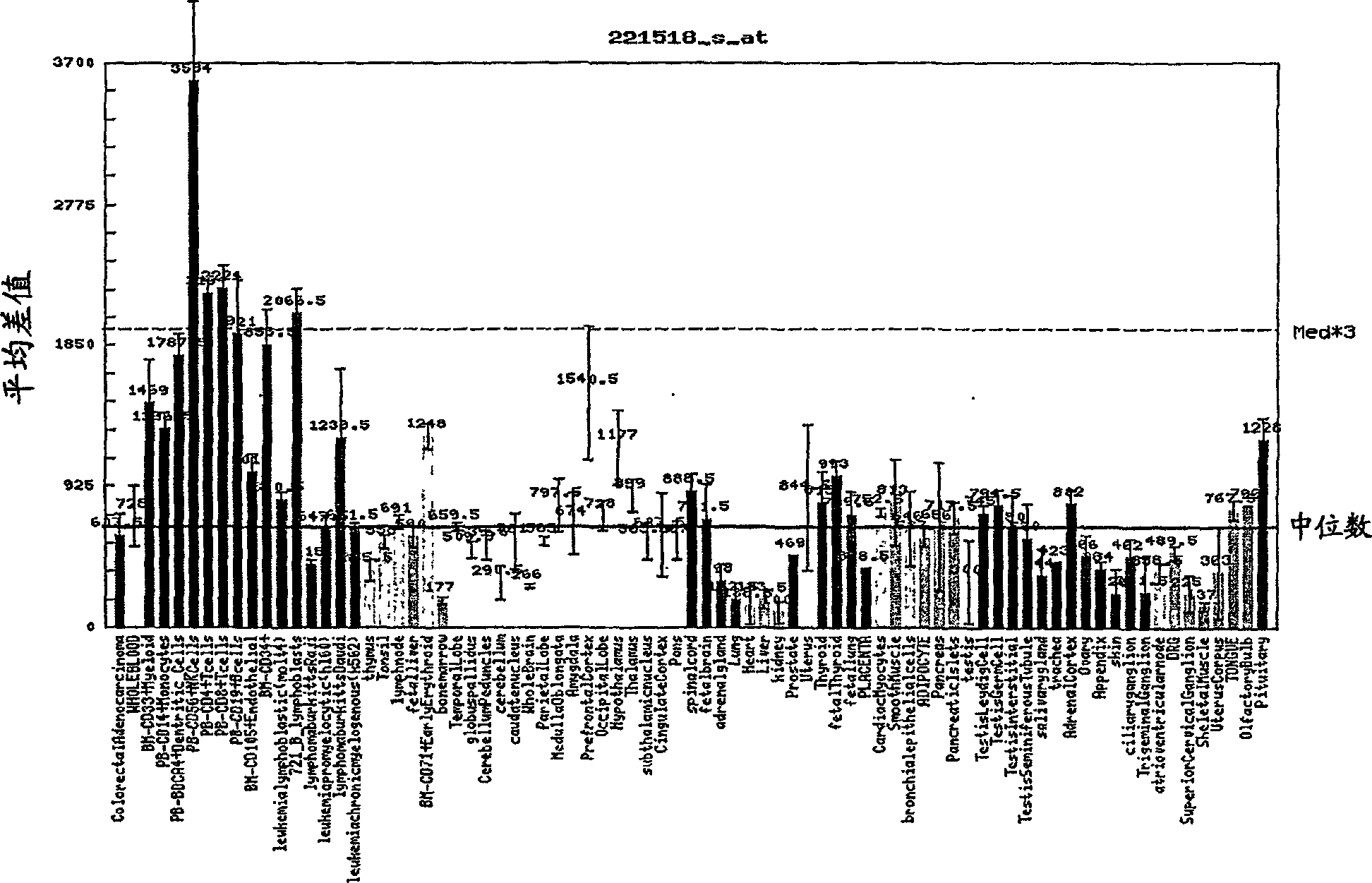
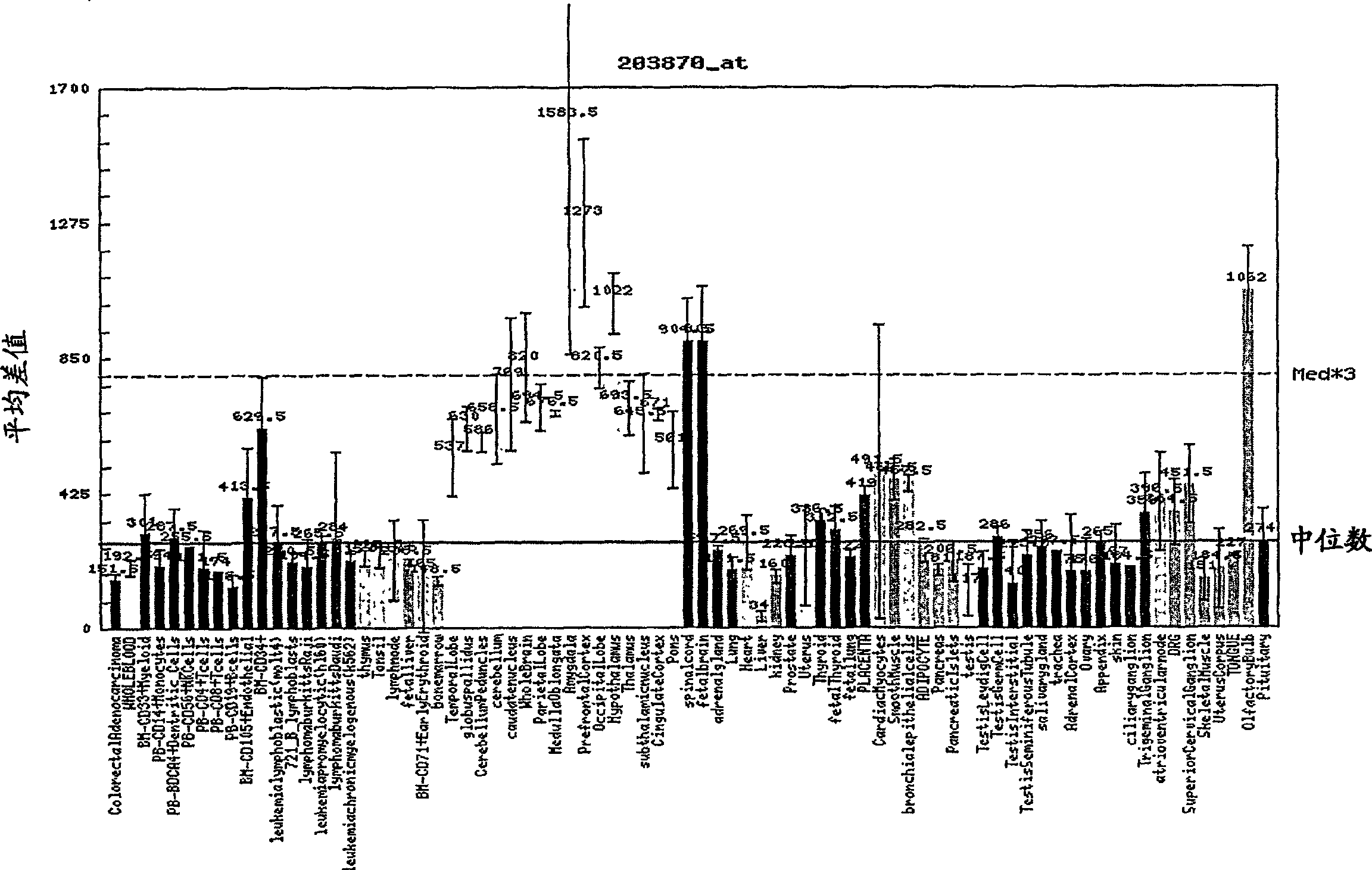
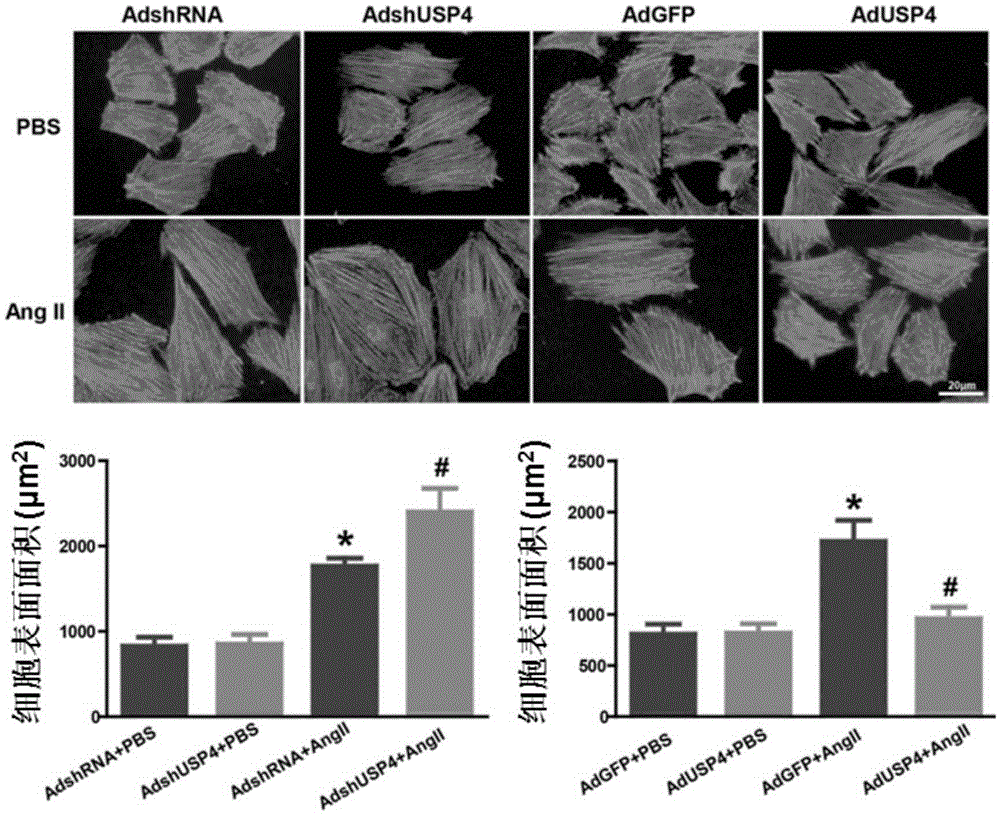
Function and application of ubiquitin specific protease 4 (USP4) in treating cardiac hypertrophy
ActiveCN105251020AProtect heart functionProtects Against Cardiac FibrosisPeptide/protein ingredientsGenetic material ingredientsCardiac fibrosisCardiac functioning
The invention discloses a function and application of ubiquitin specific protease 4 (USP4) in treating cardiac hypertrophy, and belongs to the field of gene functions and application. The mutual relation between the expression of the USP4 and cardiac hypertrophy is determined; research results show that in a cardiac hypertrophy occurrence model, the expression of the USP4 is significantly reduced compared with a normal group; by restraining the expression of the USP4, the cardiac hypertrophy and fibrosis are significantly promoted, and the cardiac function is deteriorated; by promoting the expression of the USP4, cardiac hypertrophy and fibrosis are significantly restrained, and the cardiac function is protected. Accordingly, the USP4 can be used as a target gene for screening and preparing the medicine which protects the cardiac function, resists cardiac fibrosis and / or prevents, relieves and / or treats cardiac hypertrophy, and an effective new way is provided for treating cardiac hypertrophy.
Owner:武汉惠康基因科技有限公司
Inhibitors of deubiquitinating proteases
InactiveUS20160090351A1Prevent and treat diseaseBiocidePeptide/protein ingredientsProteinase activityUbiquitinated Proteins
Disclosed are small molecule inhibitors of deubiquitinating enzymes (DUBs), and methods of using them. Certain compounds display a preference for specific ubiquitin specific proteases (USPs).
Owner:BRANDEIS UNIV
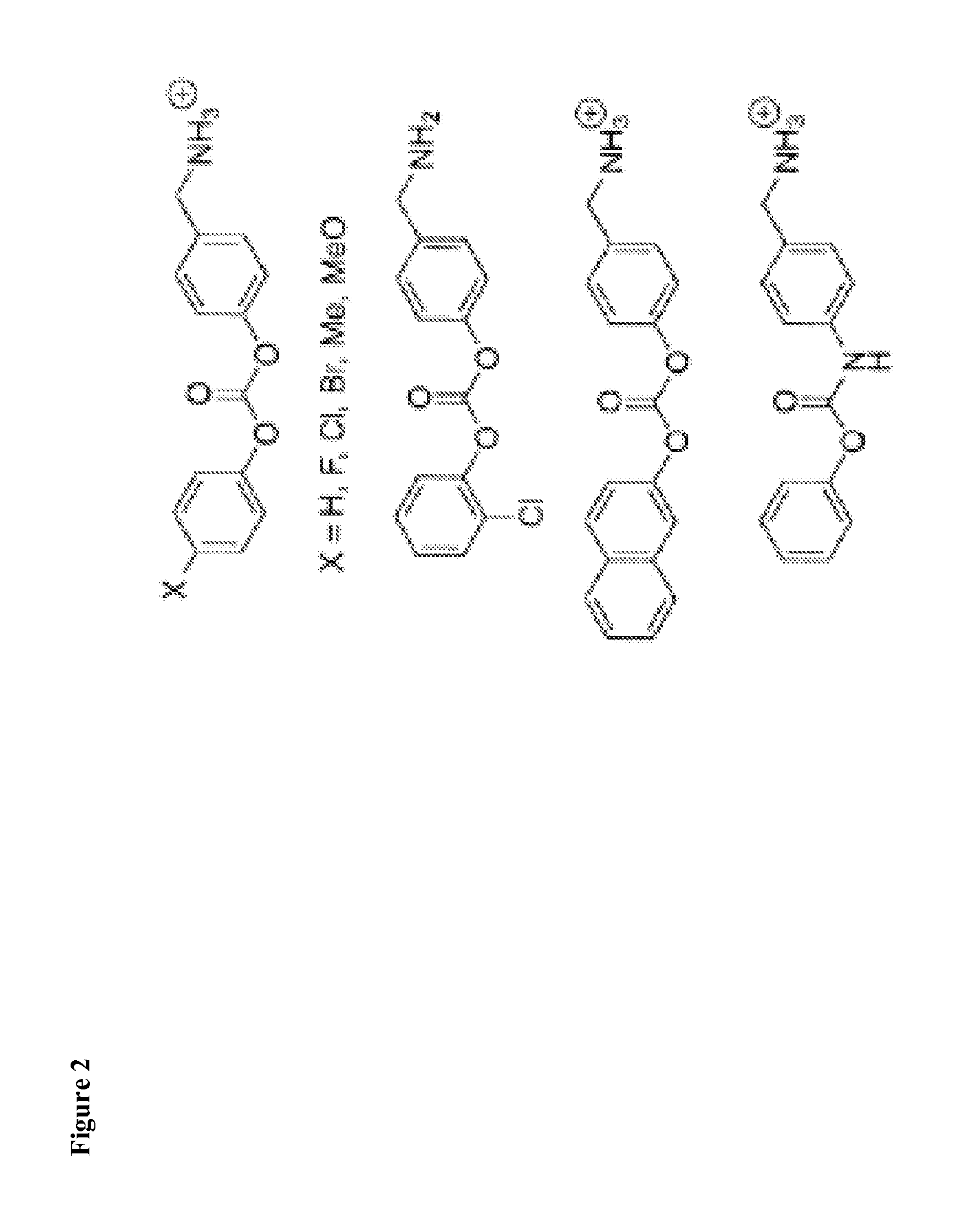
6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use
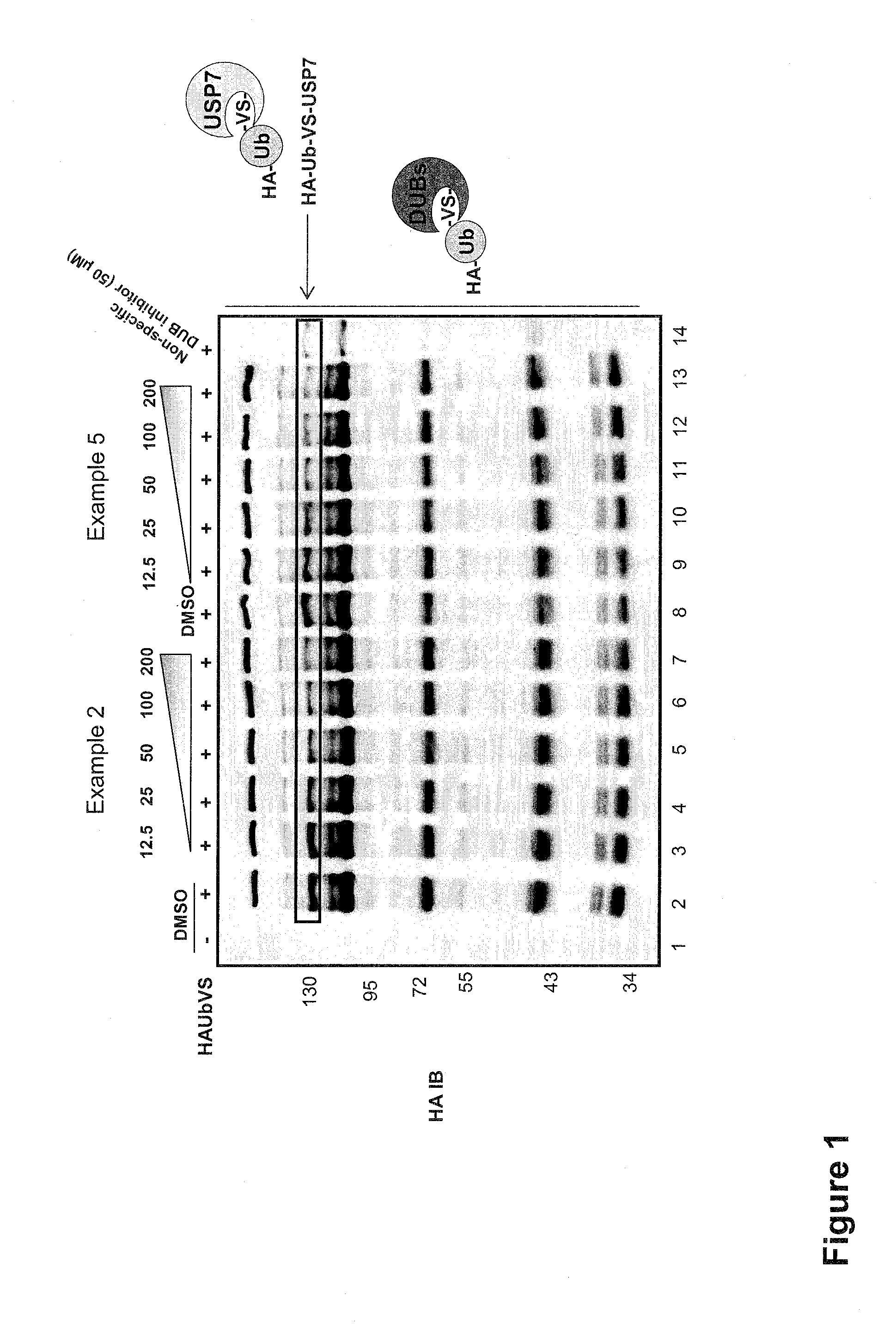
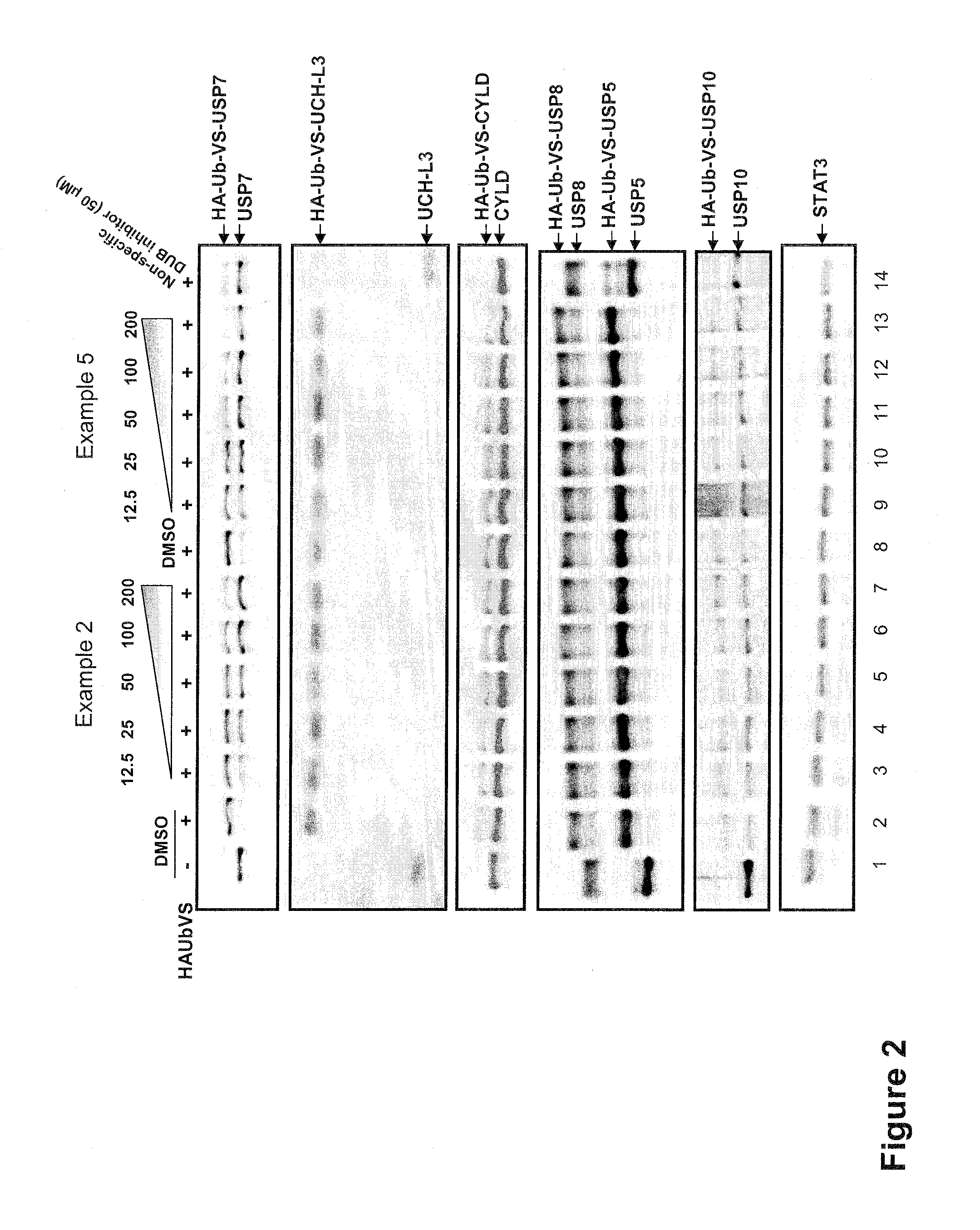
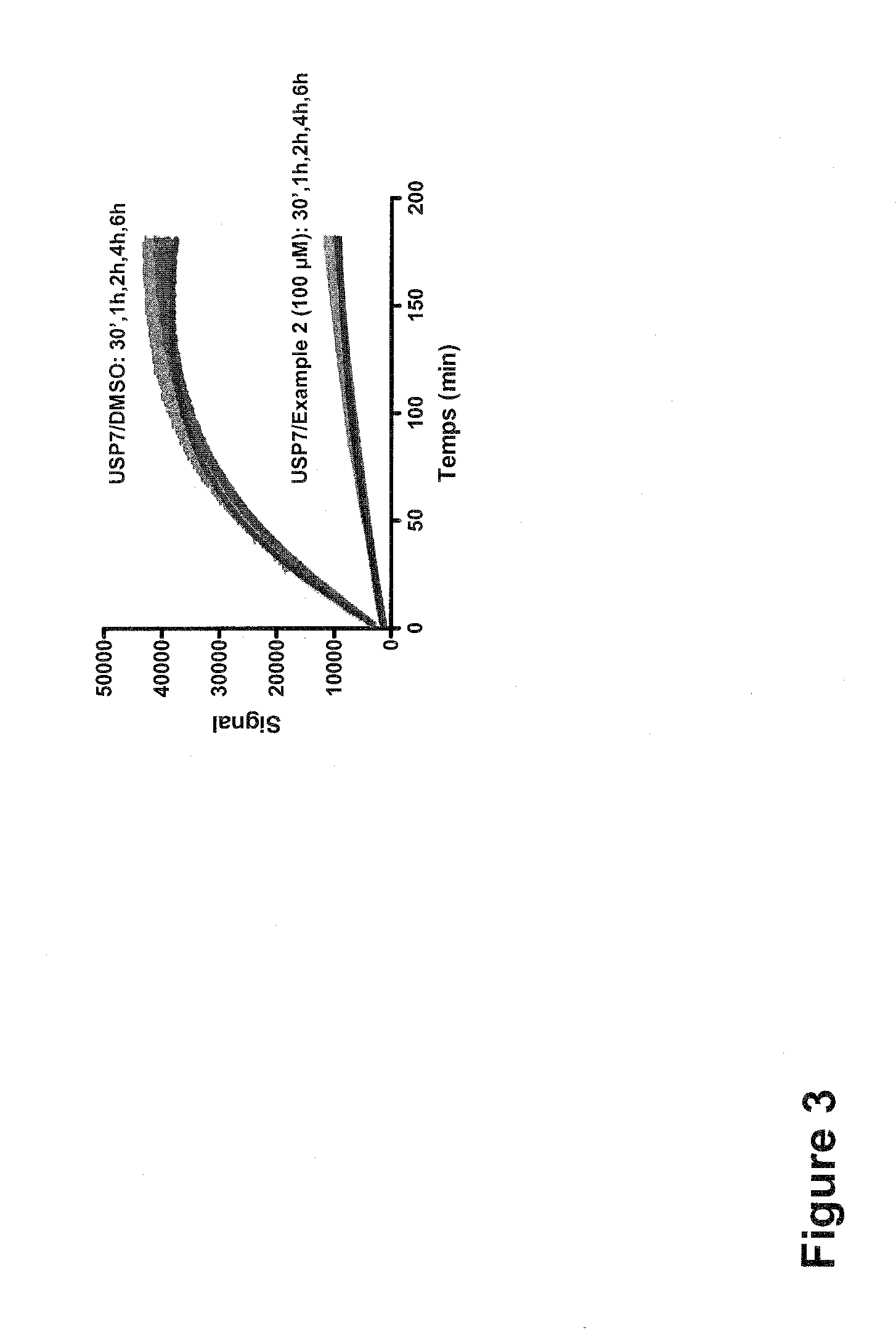
The present invention generally relates to 6-amino-2,4-dihydropyrano [2,3-c] pyrazoles as a ubiquitin specific protease 7 (USP7) inhibitor useful for the treatment of diseases mediated by malfunction of USP7, such as inflammation, cancer, and immunological disorders. The invention described herein also pertains to pharmaceutical compositions and methods for treating diseases mediated by malfunction of USP7, in mammals using compounds disclosed herein.
Owner:PURDUE RES FOUND INC
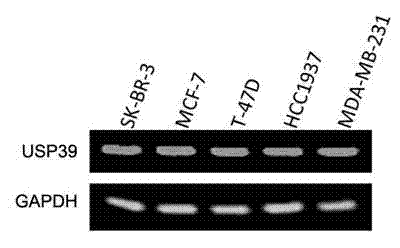
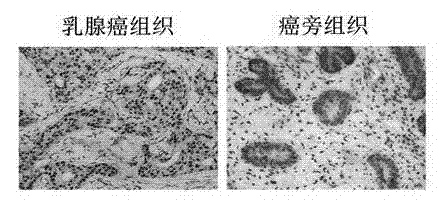
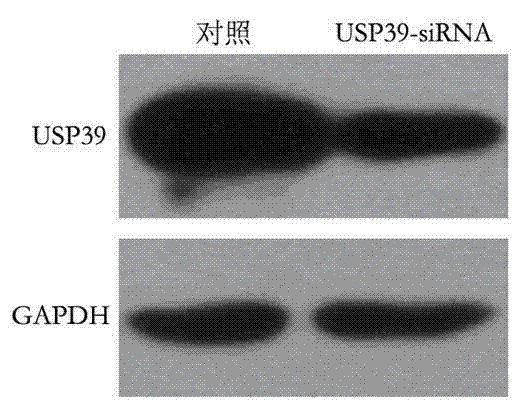
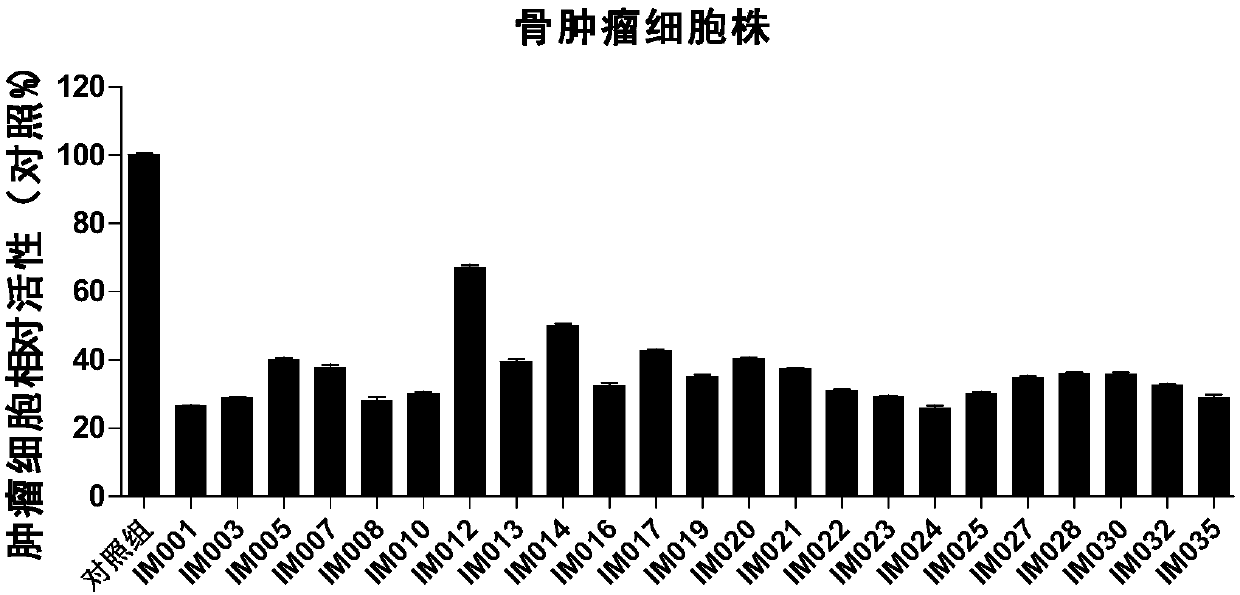
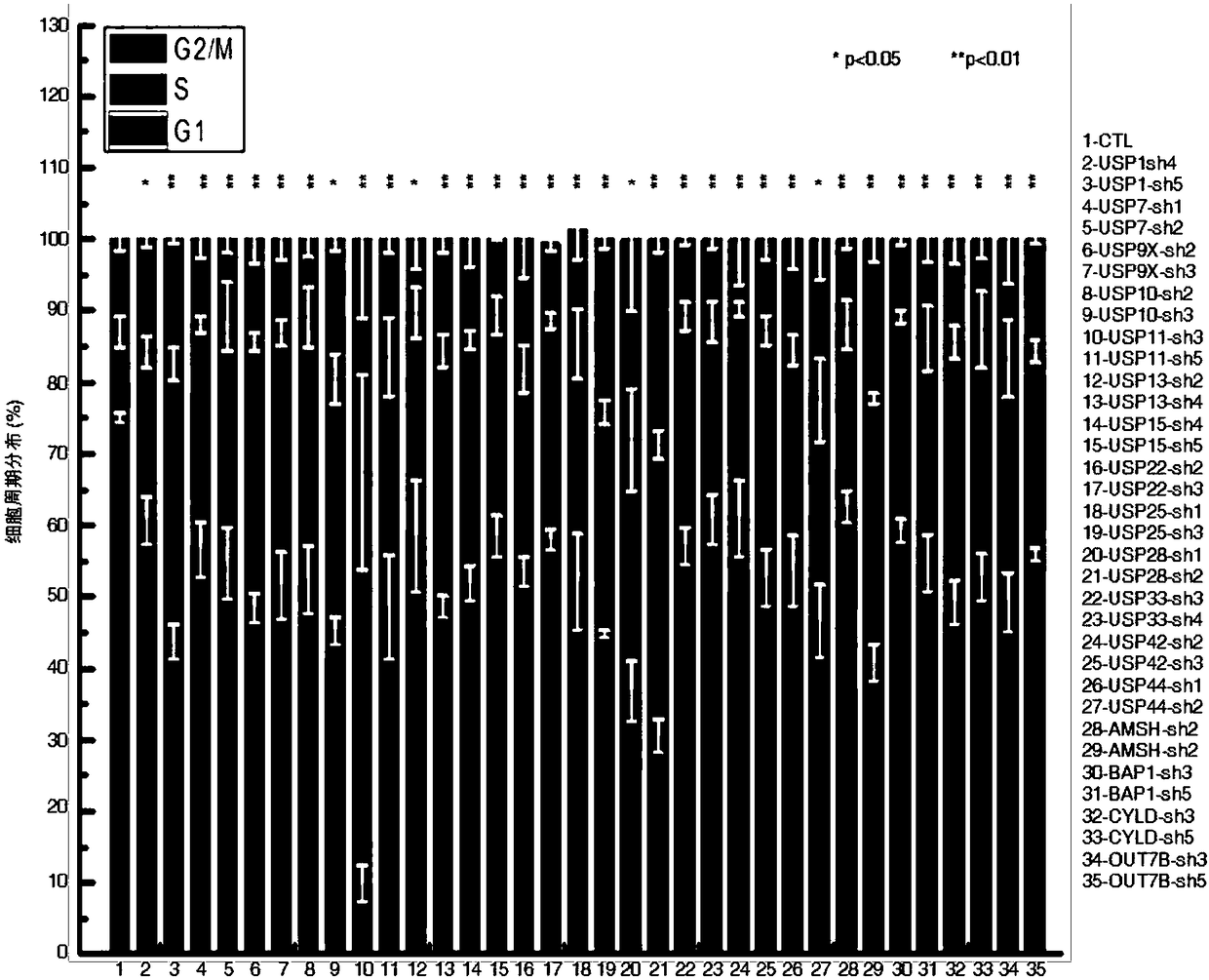
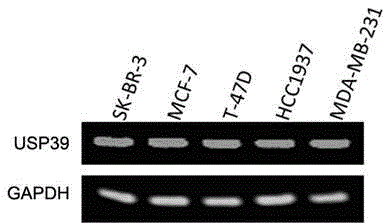
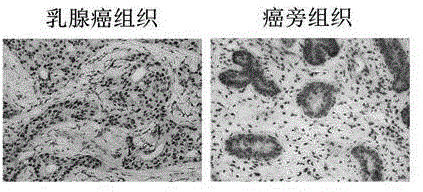
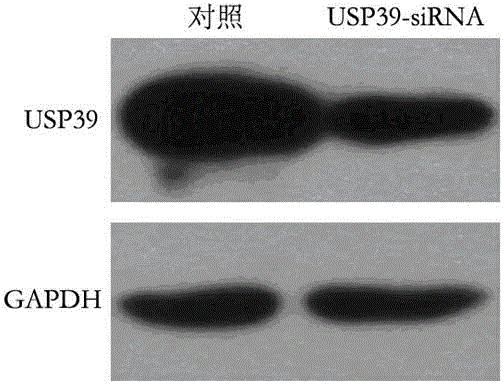
Recombinant lentiviral vector containing ubiquitin-specific protease gene USP39-shRNA (short hairpin ribonucleic acid) and application thereof
The invention provides a recombinant lentiviral vector containing ubiquitin-specific protease gene USP39-shRNA (short hairpin ribonucleic acid) and application thereof. The test proves that expression of USP39 in breast cancer tissue is obviously higher than that in normal breast tissue; the immunohistochemical detection confirms that the expression of USP39 in the breast cancer tissue is obviously higher than that in normal breast tissue; the immunohistochemical detection confirms that expression of USP39 protein in the breast cancer tissue is obviously higher than that in para-carcinoma tissue; siRNA (small interfering ribonucleic acid) is designed in a breast cancer cell system to interfere the expression of the USP39; it is found that proliferation of breast cancer cell after reducing the USP39 is obviously restrained; the cell apoptosis ratio is obviously improved; the cell ratio at the G1 stage is increased; the cell ratio at the S sage is reduced; and the clonality of the cell is obviously reduced. The test proves that the USP39 has an important role in facilitation of development of the breast cancer; and theoretical and experimental basis is provided for preparation of a drug for preventing and treating the breast cancer by the lentiviral vector containing USP39-shRNA.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Hydroxy substituted tetrahydro-beta-carboline small-molecular organic compounds, as well as derivatives and medical application thereof
The invention discloses hydroxy substituted tetrahydro-beta-carboline small-molecular organic compounds and derivatives, hydrates or pharmaceutically acceptable salts of the compounds, pharmaceuticalcomposition containing the compounds, an application of the compounds to preparation of drugs for preventing and / or treating various USP (ubiquitin-specific protease)15-mediated malignant tumors and tumor metastasis related diseases.
Owner:EAST CHINA NORMAL UNIV
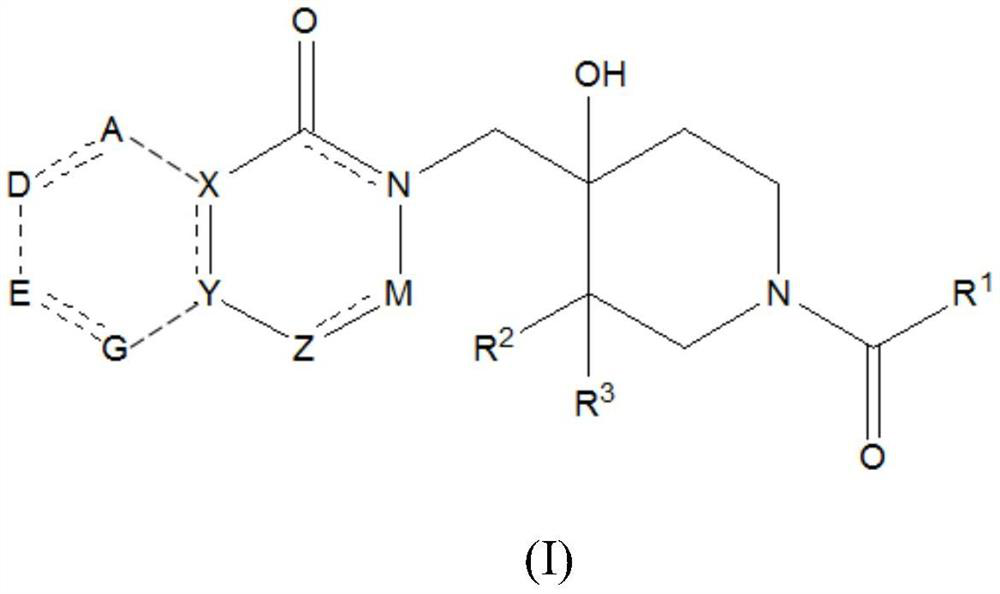
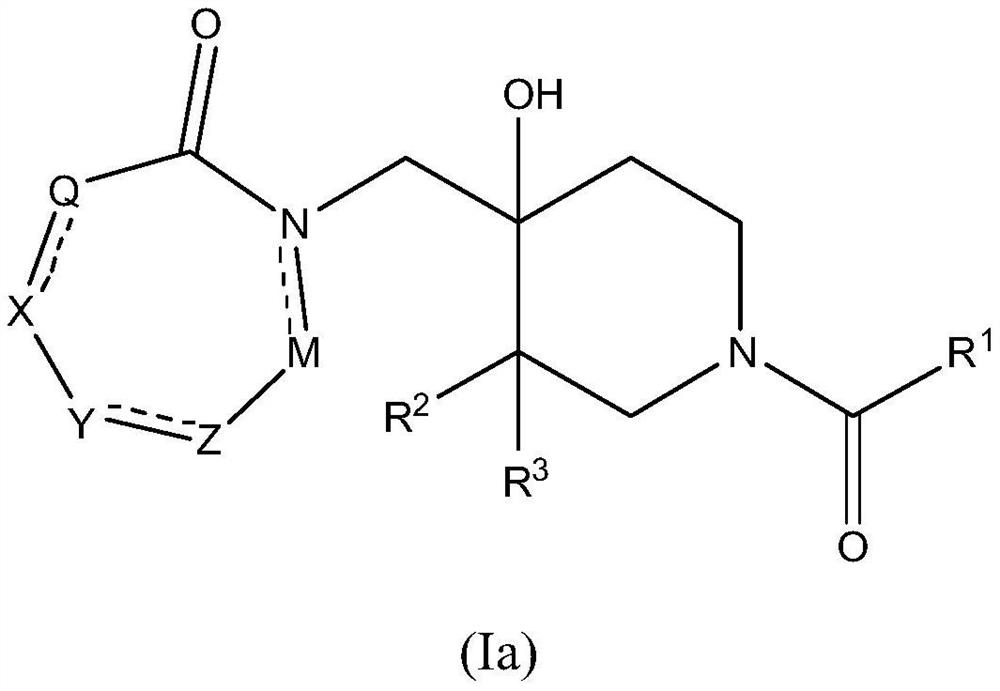
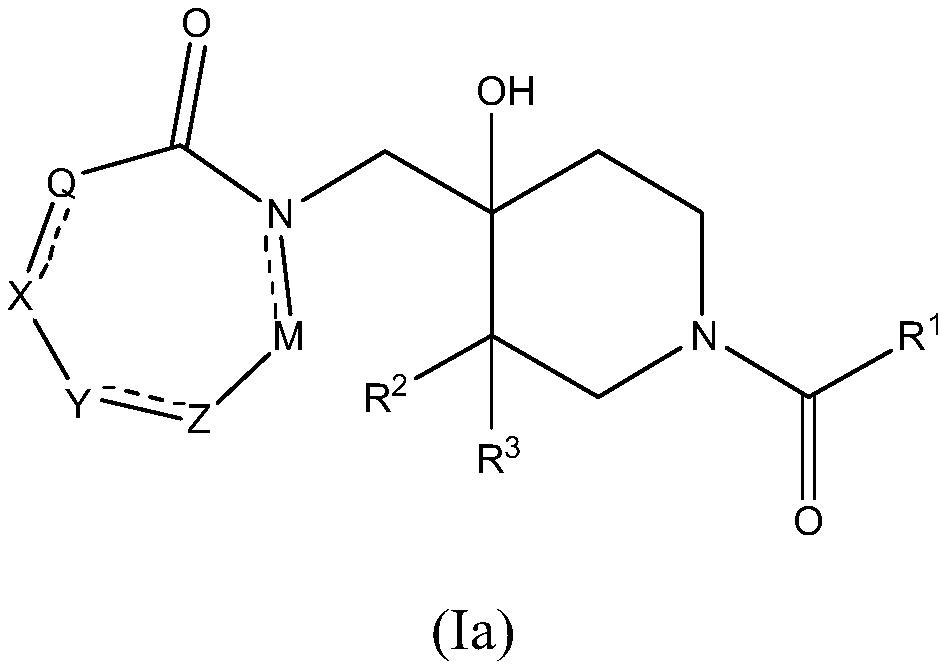
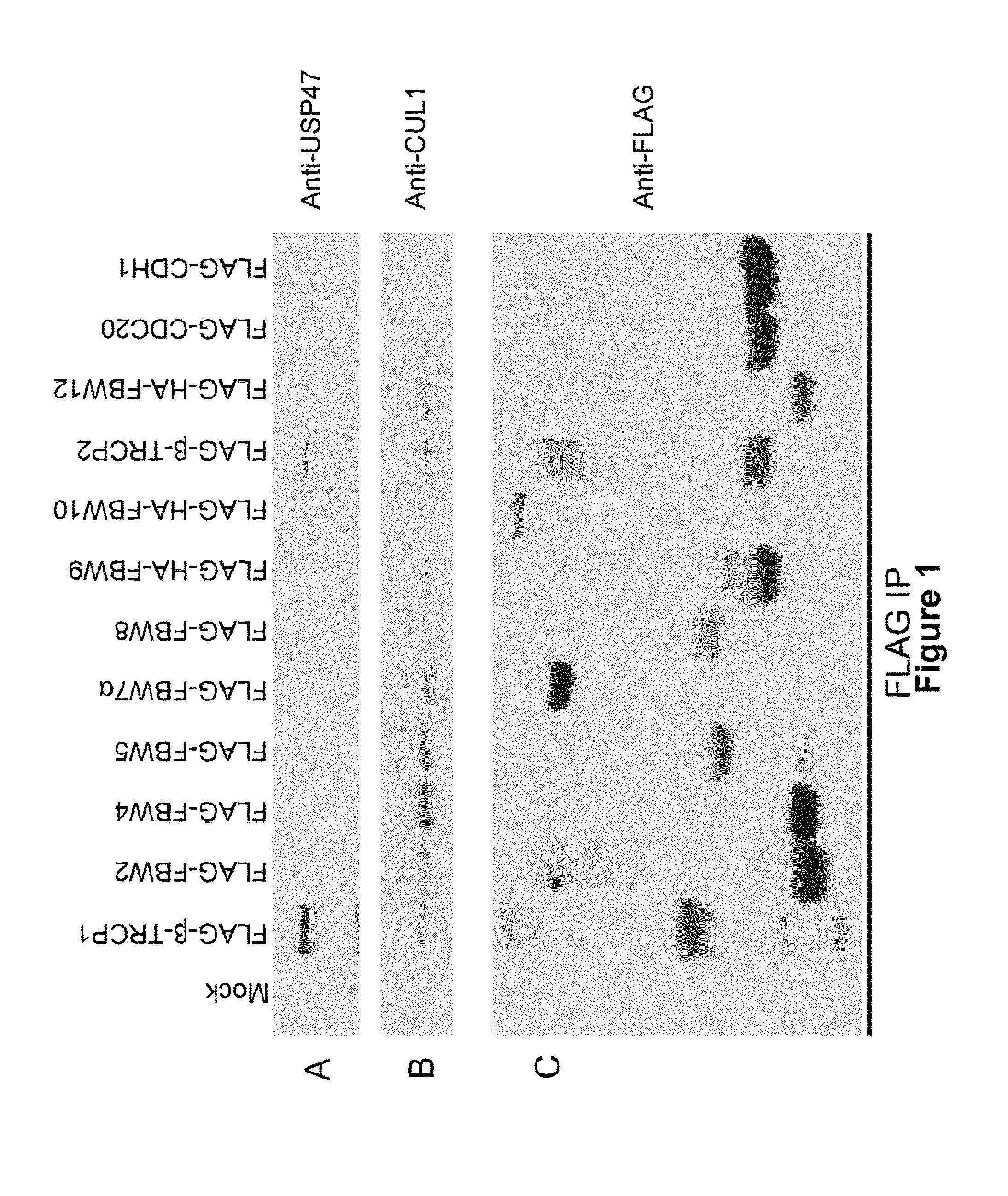
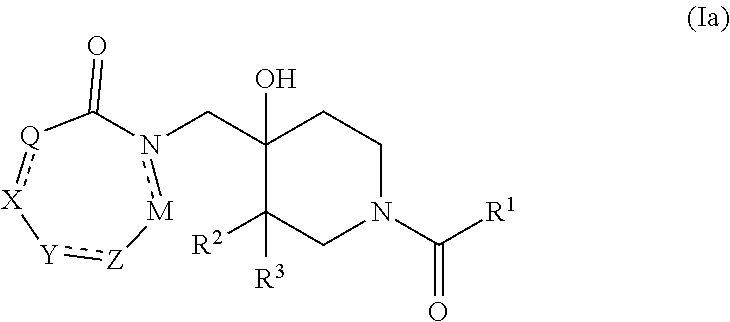
Piperidine derivatives as inhibitors of ubiquitin specific protease 7
ActiveUS10766903B2Organic active ingredientsOrganic chemistryUbiquitin-Specific ProteasesUbiquitin specific protease
Owner:ALMAC DISCOVERY LIMITED
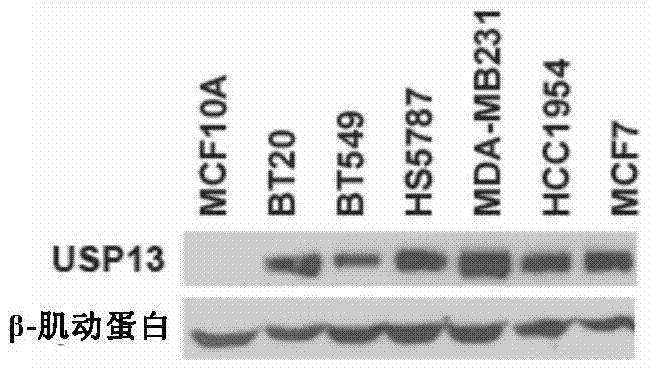
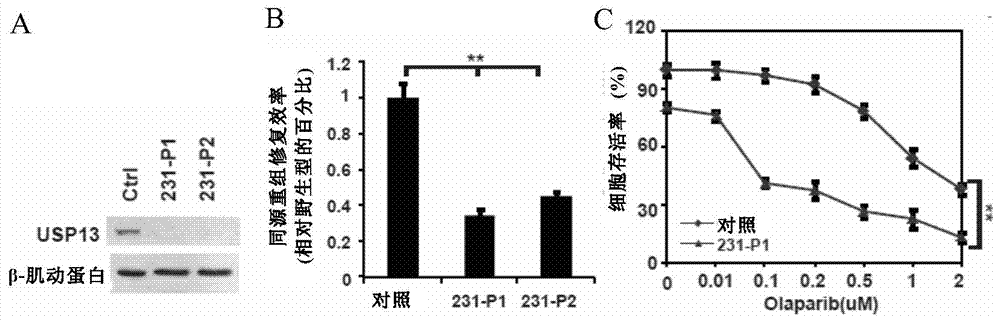
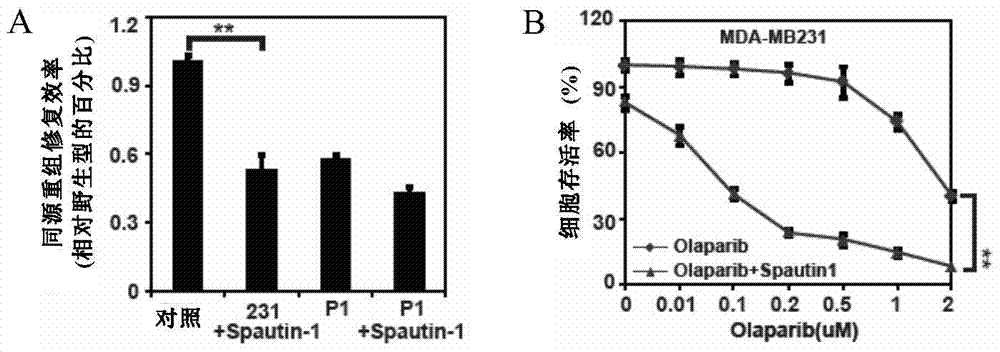
Pharmaceutical composition containing USP13 (ubiquitin-specific proteases 13) inhibitor and PARP (poly ADP-ribose polymerase) inhibitor and application thereof
InactiveCN106975079AAntineoplastic agentsHeterocyclic compound active ingredientsPARP inhibitorWilms' tumor
The invention provides a pharmaceutical composition containing a USP13 inhibitor and a PARP inhibitor and application thereof. Specifically, the invention provides a pharmaceutical composition which contains the active constituent (a) USP13 inhibitor, the active constituent (b) PARP inhibitor, and (c) a pharmaceutically acceptable carrier. The USP13 inhibitor can remarkably improve the anti-tumor effect of the PARP inhibitor, and the homologous recombination repair efficiency of tumor cells can be reduced remarkably through combination of the two inhibitors, so that tumor growth can be suppressed more effectively. The pharmaceutical composition can effectively treat cancer.
Owner:SHANGHAI EAST HOSPITAL
Method for identifying modulators of g3bp activity
A method of identifying a lead or candidate compound that modulates the activity of GTPase-Activating Protein SH3 Domain-Binding Proteins (G3BP) is provided, which includes determining whether a compound modulates the interaction between the N-terminal Nuclear Transport Factor 2-like (NTF2L) domain of G3BP and FGDF peptide of ubiquitin specific protease 10 (USP10) or non-structural protein 3 (nsP3).
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Ubiquitin-specific protease occurring in the brain and dna encoding the same
A ubiquitin-specific protease occurring in the brain and a DNA encoding it, which are useful for research on the molecular mechanism of the neuroplasticity expression and so on.
Owner:EISIA R&D MANAGEMENT CO LTD
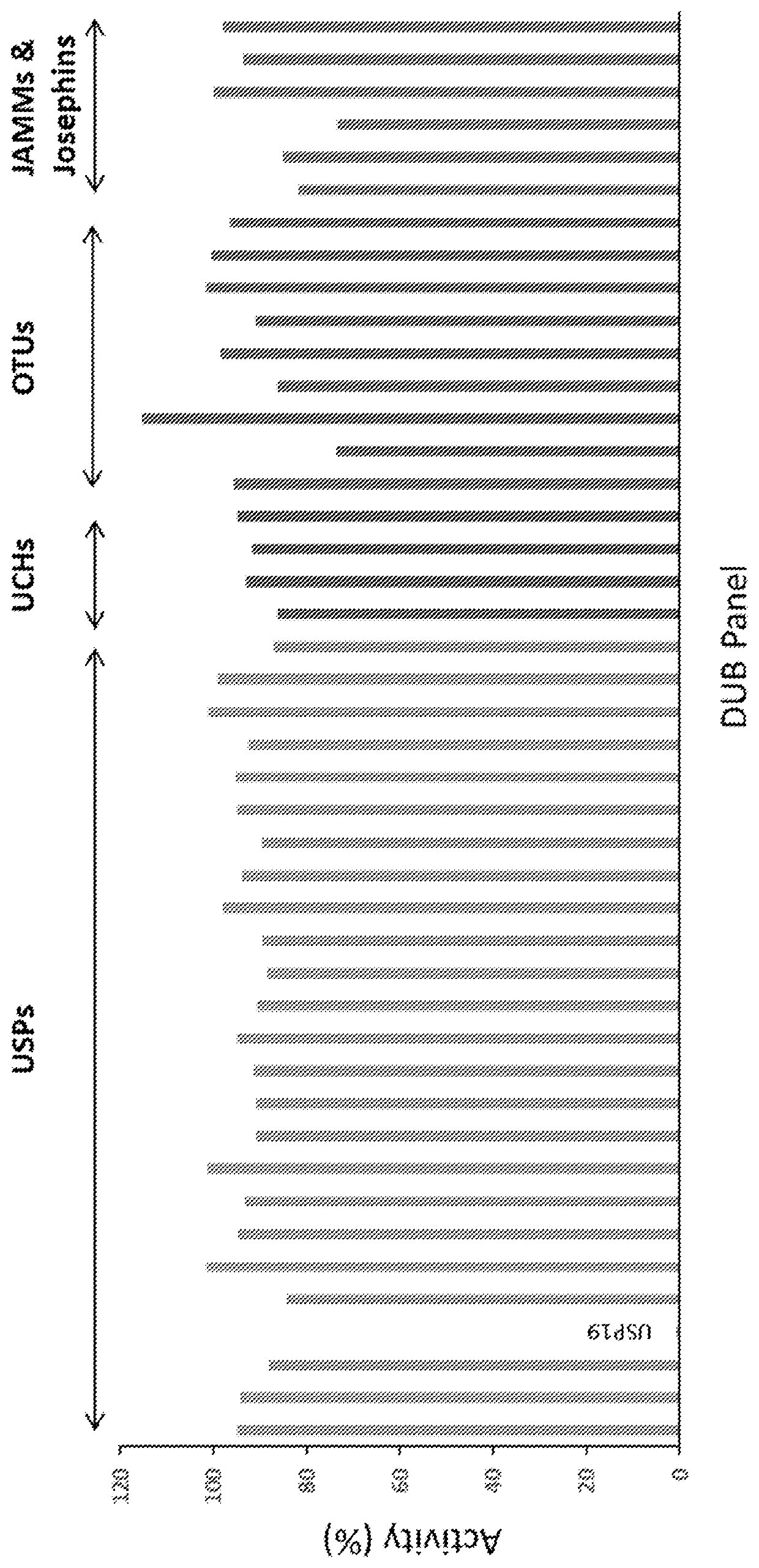
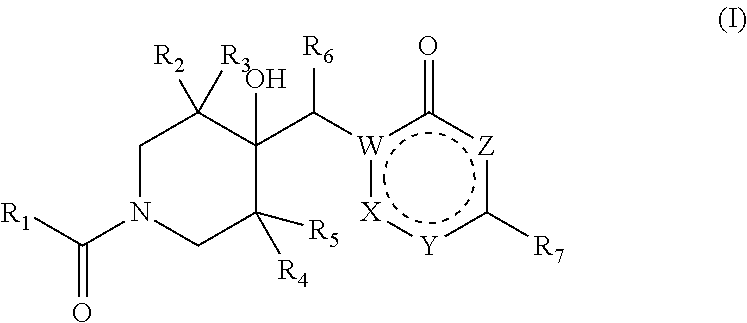
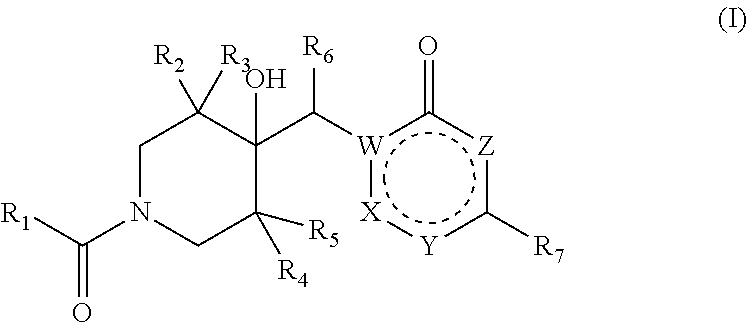
4-hydroxypiperidine derivatives and their use as inhibitors of ubiquitin specific protease 19 (USP19)
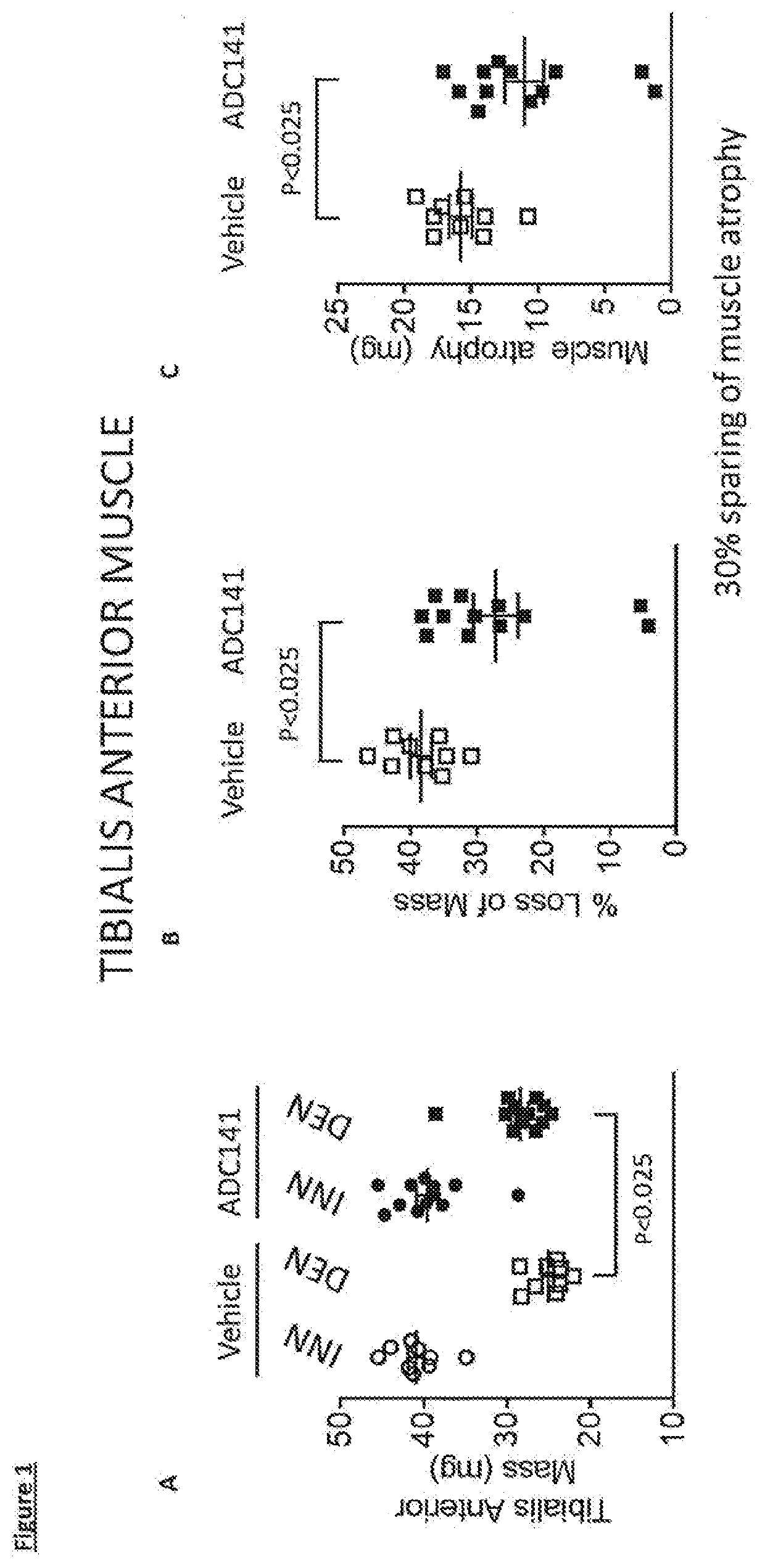
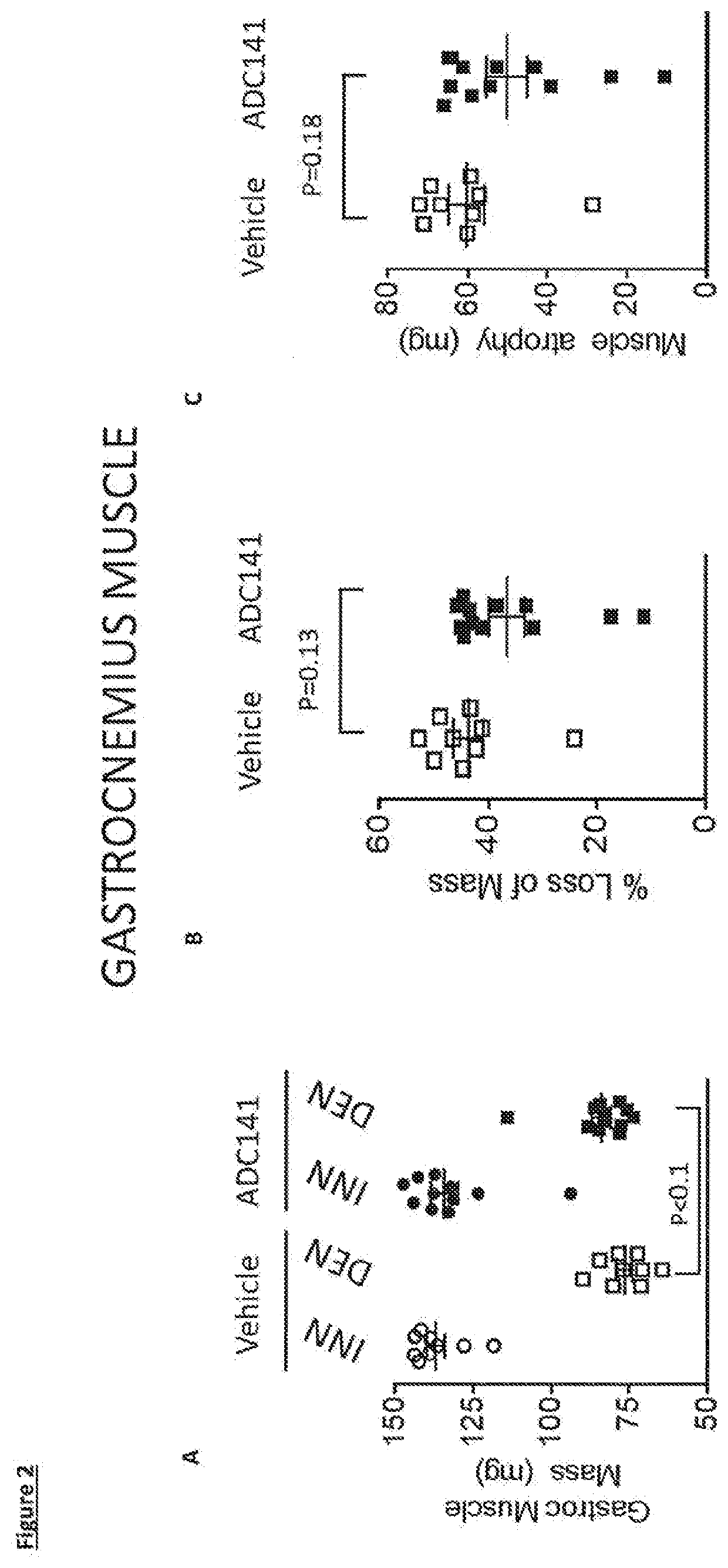
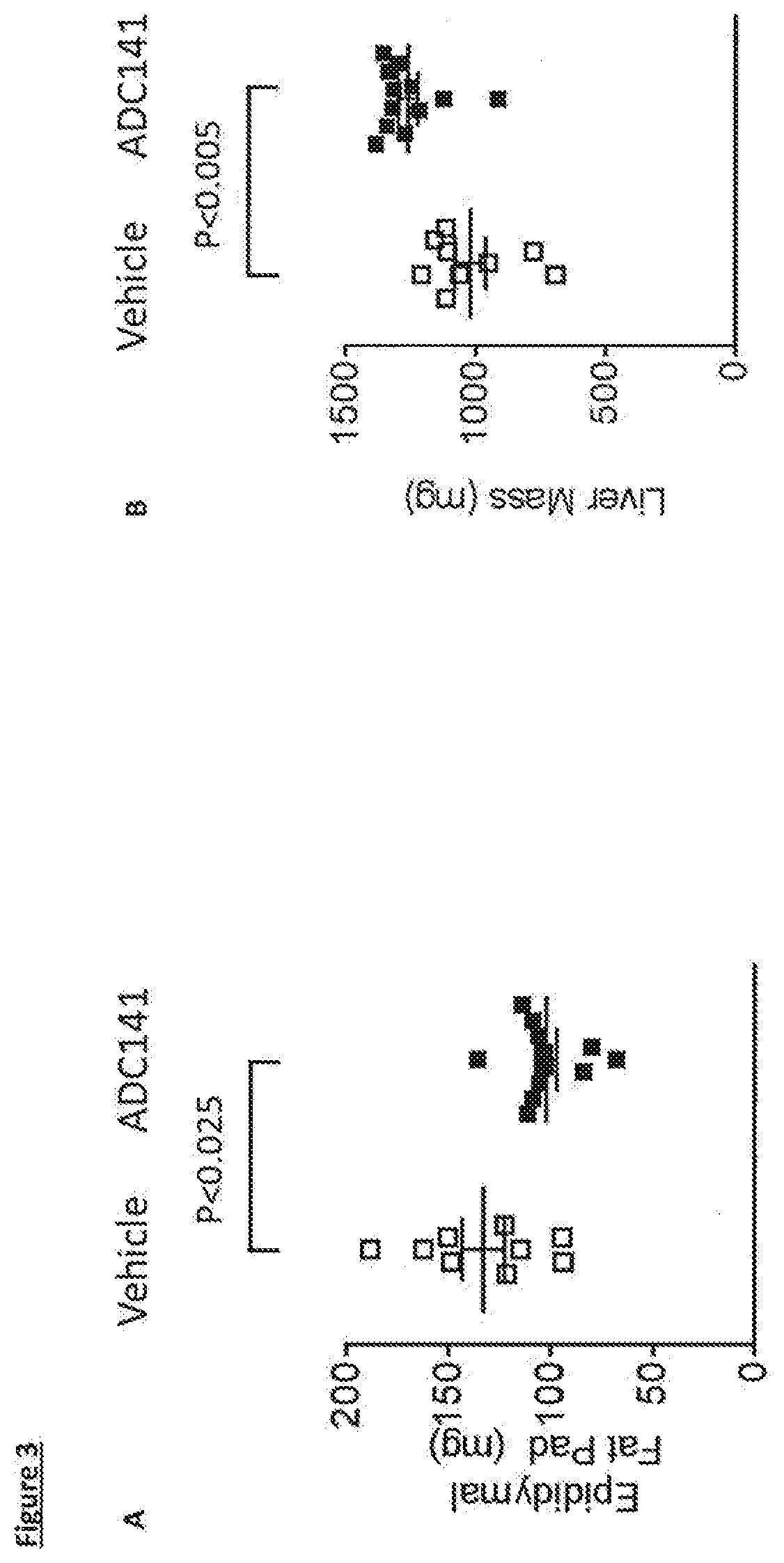
Inhibitors of ubiquitin specific protease 19 (USP19) of Formula (I) are provided, together with pharmaceutical compositions comprising said inhibitors, and methods of use thereof. The compounds can beused in in the treatment of muscular atrophy, obesity, insulin resistance or type II diabetes or in reducing the loss of muscle mass.
Owner:ALMAC DISCOVERY LIMITED
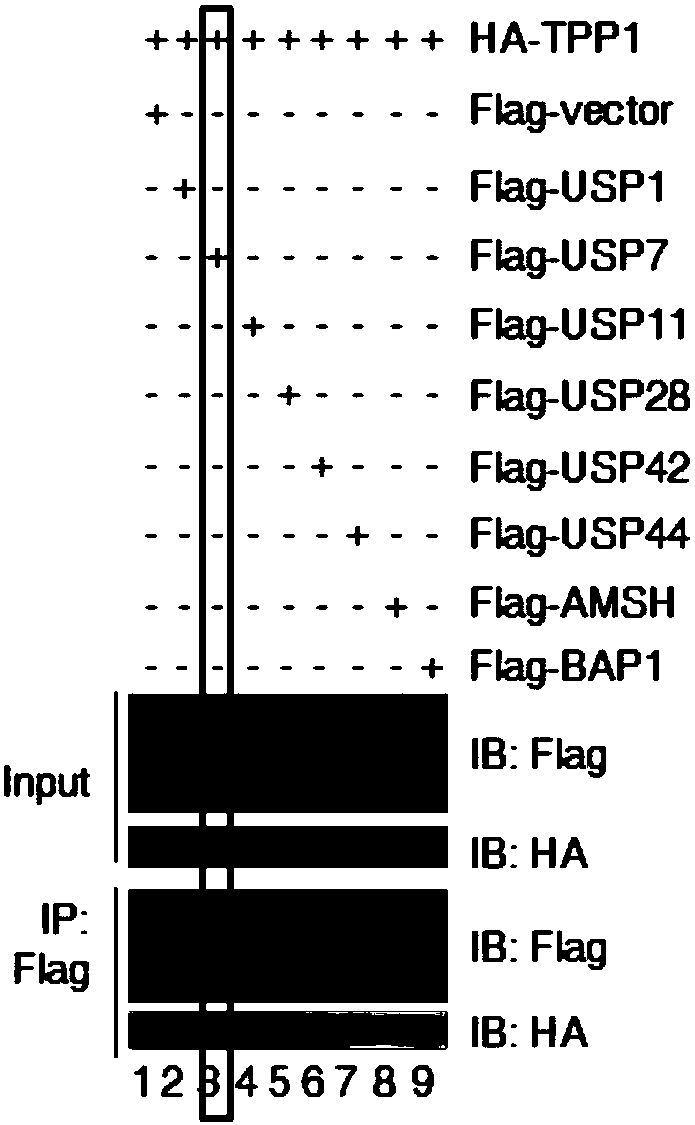
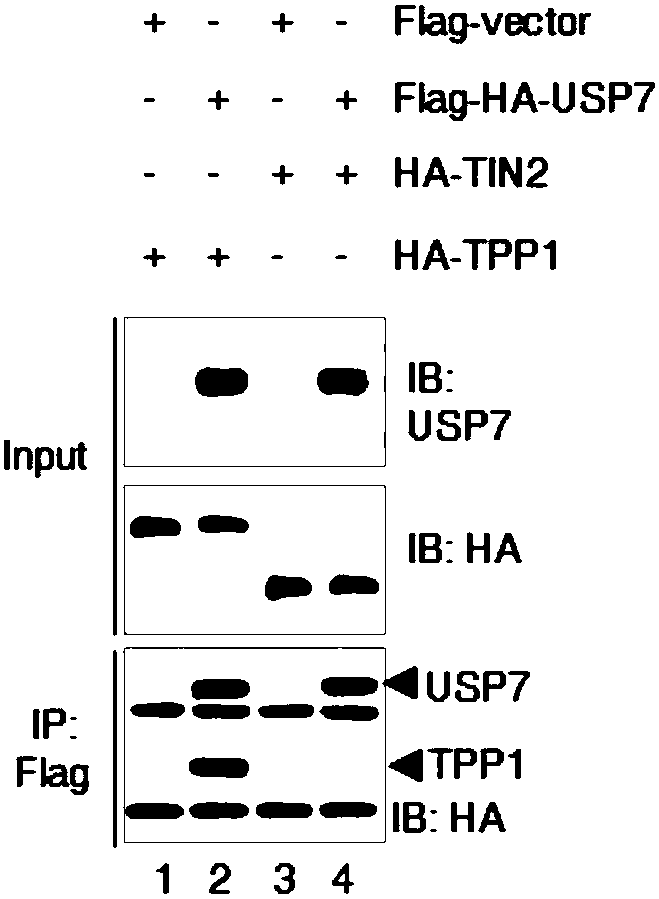
Medical application of ubiquitin-specific proteases USP 7 to treating aging and related diseases
ActiveCN108144050AAvoid degradationPrevents telomere damageNervous disorderPeptide/protein ingredientsDiseaseLymphatic Spread
The invention provides application of ubiquitin-specific proteases USP7 to preparing drugs for treating aging and related diseases. The amino acid sequence of the USP7 is shown as SEQ. NO.2. Accordingto the application of the ubiquitin-specific proteases USP7 to preparing the drugs for treating aging and related diseases, the USP7 is activated to avoiding degradation of telomere proteins and damage of telomeres, particularly degradation of telomere proteins in environmental stress states such as radiation damage or oxidative stress., and meanwhile, avoid telomere damage and shortening, tissueand organ aging and related diseases caused by pressurized or stressed anaerobic states; expression and enzyme activity of the USP7 is inhibited to prevent protecting effects of the USP7 on the telomere proteins and the telomeres, to lead to telomere shortening and damage response, and further to cause proliferation and growth inhibition, aging and death of tumor cells and inhibit generation, development, growth, infiltration and metastasis of tumor. The USP7 can be applied to preparing drugs for preventing and treating telomere function related diseases and provide new anti-aging thoughts and methods.
Owner:HANGZHOU NORMAL UNIVERSITY
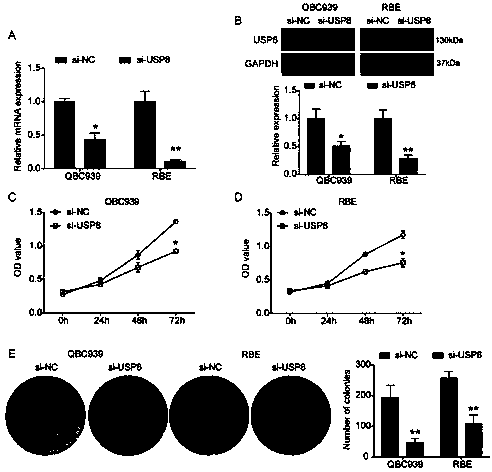
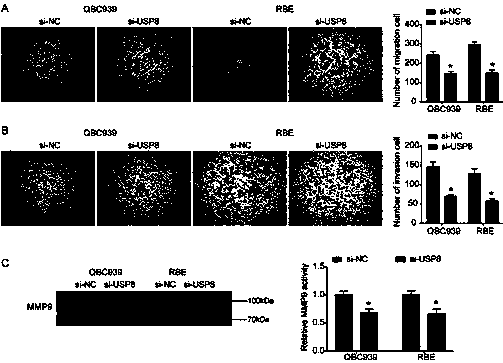
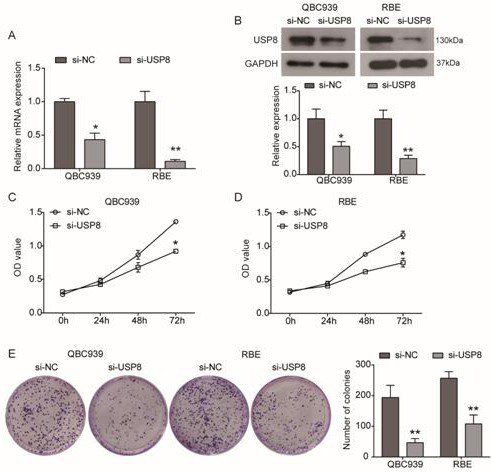
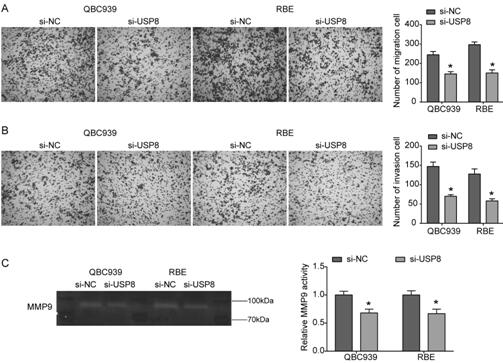
Cholangiocarcinoma detection, treatment and prognosis target point and application
ActiveCN110699453AReduced activityPrevent proliferationOrganic active ingredientsHydrolasesNucleotideOncogene
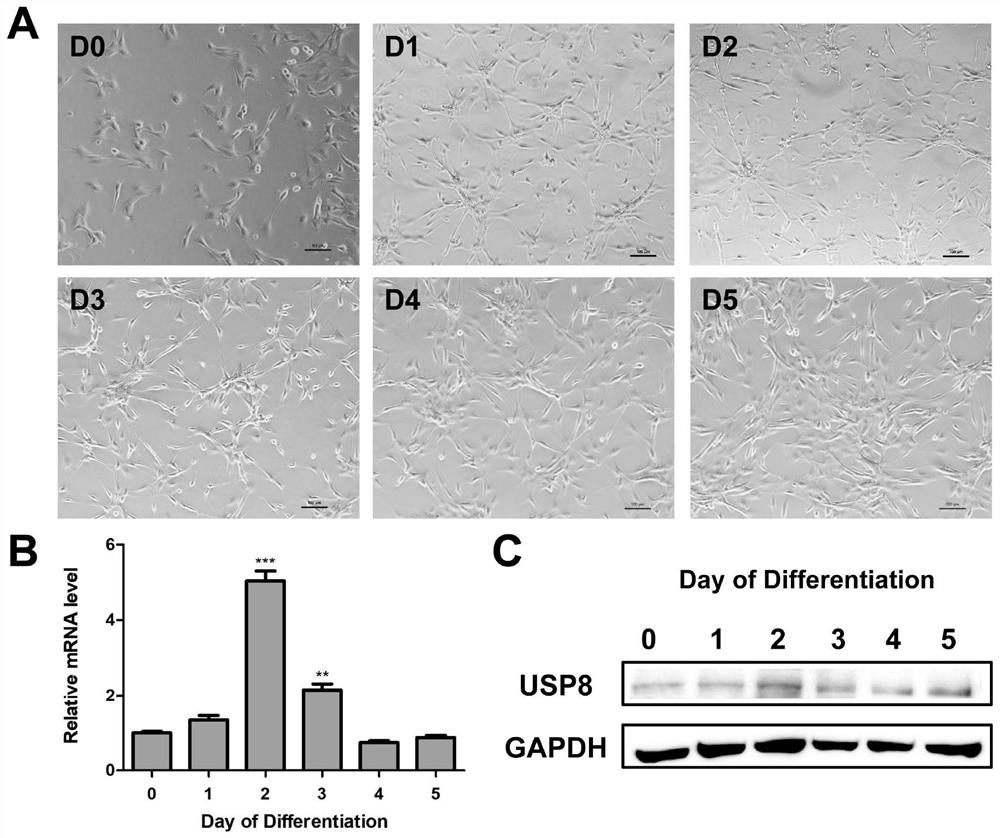
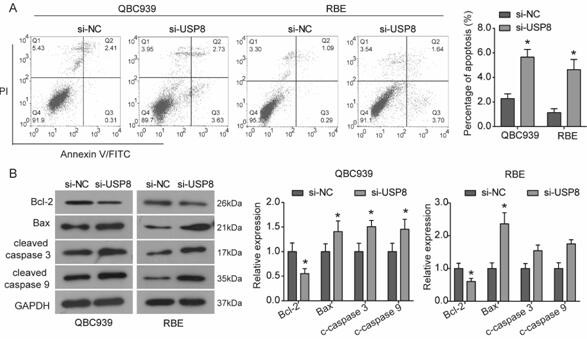
The invention relates to a cholangiocarcinoma cell medicine target point. The medicine target point is ubiquitin specific protease 8, and the nucleotide sequence is shown as SEQID NO:1 in a sequence table. For the first time, the invention puts forward that USP8 is knocked down, so that proliferation, migration and invasion of cholangiocarcinoma cells can be notably restrained, MMP9 activity achieving important effects on cell adhesion, neoplasm invasiveness and transfer is reduced, and the USP8 is promoted to achieve effect of oncogene for growth and transfer ability of cholangiocarcinoma. New possibility is provided for development of cholangiocarcinoma medicines and cholangiocarcinoma prevention, treatment and prognosis.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Usp7 inhibition
Owner:DANA FARBER CANCER INST INC
Application of ubiquitin-specific protease 8 in regulation and breeding of Hu mutton
The invention relates to the application of ubiquitin-specific protease 8 (USP8) in regulating the meat quality and breeding of Hu mutton. Experiments have found that USP8 can affect the eating quality of Hu sheep muscle. The higher the expression level of USP8, the better the eating quality of Hu sheep. Therefore, Hu sheep with good meat quality can be selected as breeding sheep by screening the expression level of USP8. The invention clarifies that USP8 has an inhibitory effect on the early differentiation process of muscle satellite cells, provides a basic theoretical basis for the further protection and breeding of Hu sheep breeds, and also provides some references and technical reserves for the future directional selection of new strains .
Owner:ZHEJIANG UNIV
Pharmaceutical compounds and their use as inhibitors of ubiquitin specific protease 19 (USP19)
Provided are USP19 inhibitors, methods of treating obesity, metabolic syndrome and / or diabetes using the USP19 inhibitor compounds, as well as those compounds for use in methods of treating obesity, metabolic syndrome and / or diabetes. Also provided are methods of treating muscular atrophy, for example cachexia or sarcopenia with USP19 inhibitor compounds, plus those compounds for use in methods of treating muscular atrophy.
Owner:ALMAC DISCOVERY LIMITED
Pharmaceutical compounds and their use as inhibitors of ubiquitin specific protease 19 (USP19)
PendingUS20220033397A1Inhibitory activityUsing treatment methodOrganic active ingredientsOrganic chemistryAtrophyDrug compound
Provided are USP19 inhibitors, methods of treating obesity, metabolic syndrome and / or diabetes using the USP19 inhibitor compounds, as well as those compounds for use in methods of treating obesity, metabolic syndrome and / or diabetes. Also provided are methods of treating muscular atrophy, for example cachexia or sarcopenia with USP19 inhibitor compounds, plus those a compounds for use in methods of treating muscular atrophy.
Owner:ALMAC DISCOVERY LIMITED
Ubiquitin-specific protease occurring in the brain and DNA encoding the same
Owner:EISIA R&D MANAGEMENT CO LTD
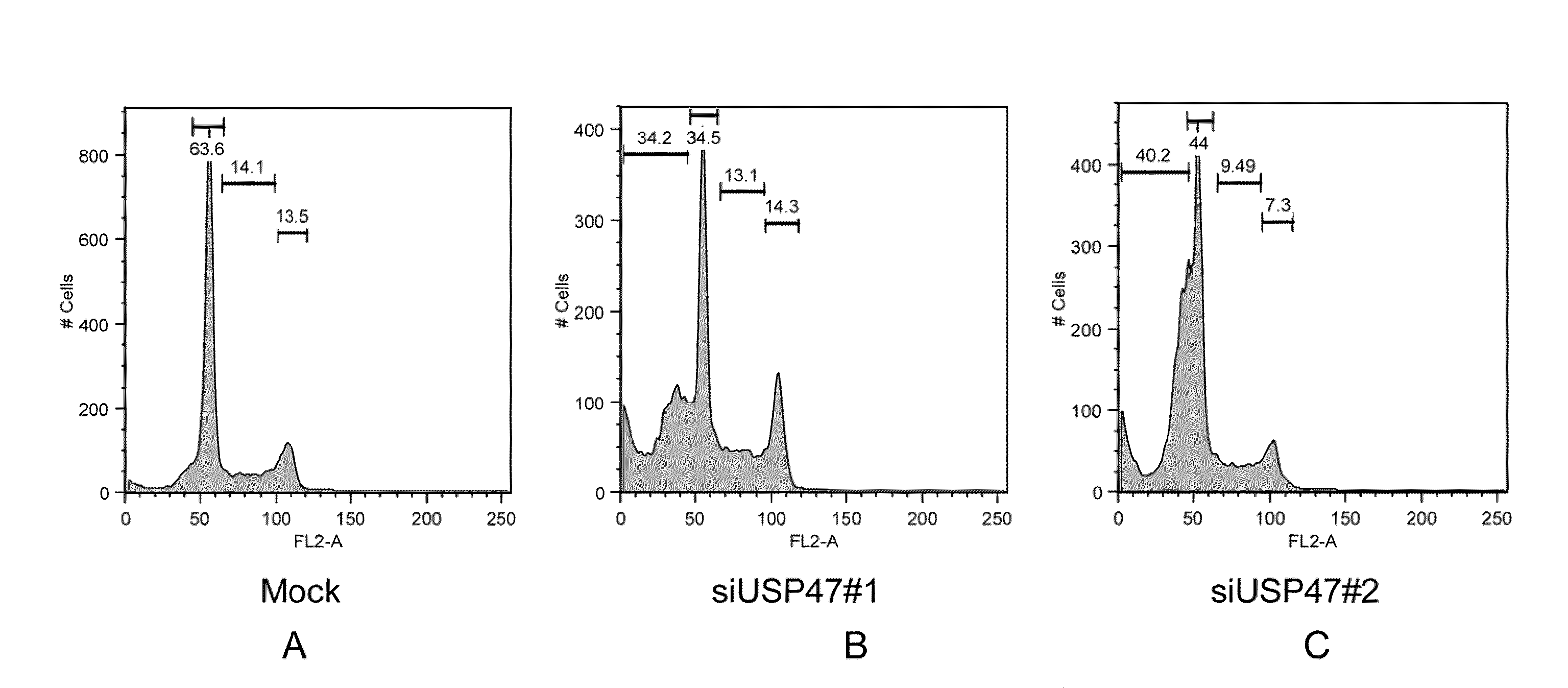
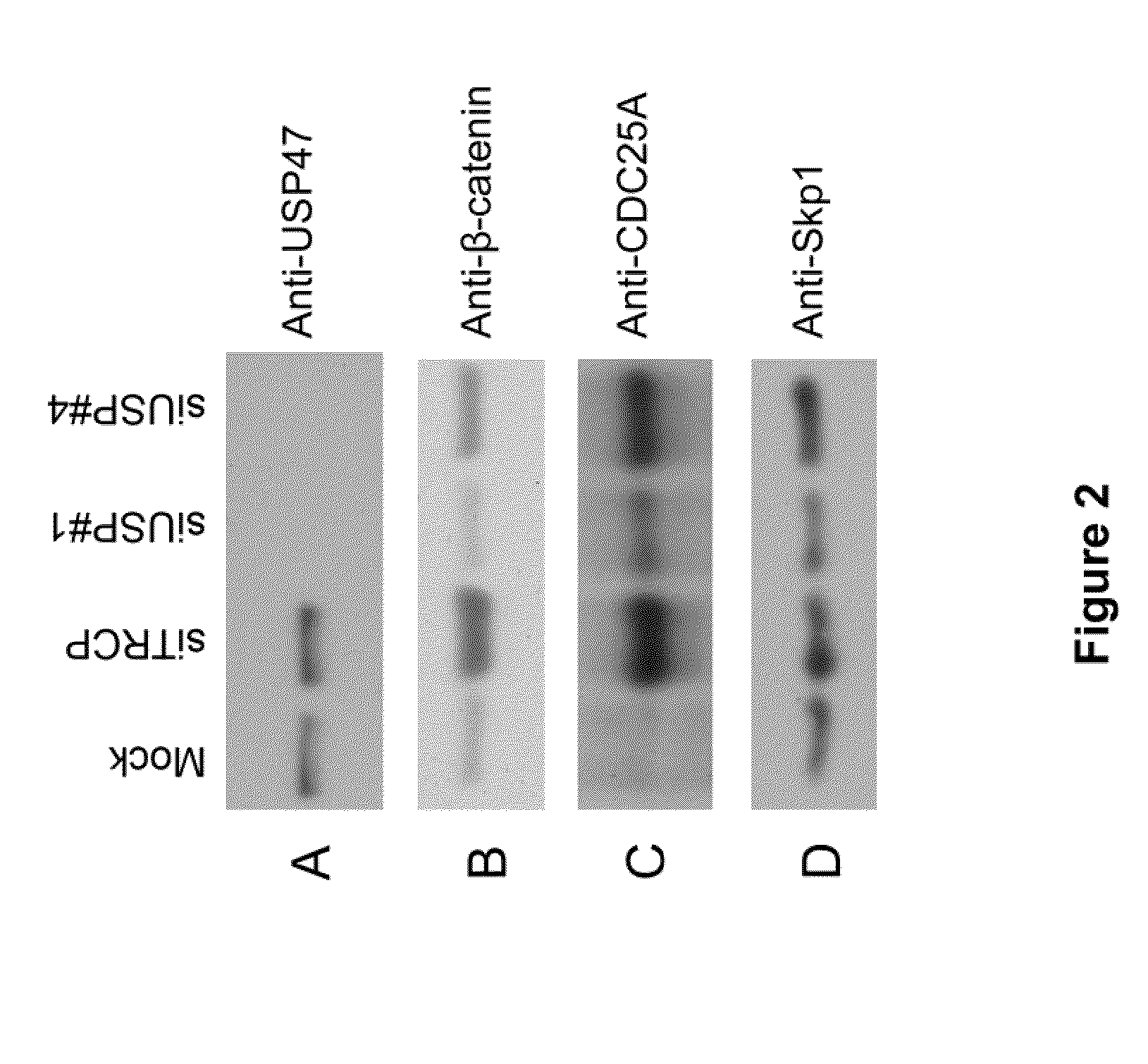
Usp47 inhibtors and methods to induce apoptosis
ActiveUS20090275639A1Improve degradation rateCompound screeningOrganic active ingredientsApoptosisBiology
The present invention relates to USP47 (ubiquitin specific protease 47) inhibitors and methods for inducing apoptosis or cell death in a target cell. In certain embodiments, the invention relates to methods and kits to screen for related agents that induce apoptosis. Additionally, the invention relates to assays for screening compounds capable of acting as USP47 inhibitors.
Owner:NEW YORK UNIV
Use of usp7 inhibitors for the treatment of acute myeloid leukemia (AML)
PendingUS20220125760A1Positively correlatedHigh levelOrganic active ingredientsAntineoplastic agentsCytarabinePharmacometrics
Resistance of acute myeloid leukemia (AML) cells to DNA damaging therapeutic agents is dependent on CHK1 protein levels. Here, the inventors demonstrate that in AML, CHK1 protein stability relies on the expression and activity of Ubiquitin Specific Protease 7 (USP7). CHK1 and USP7 levels are positively correlated in AML cell lines and primary patient specimens with high CHK1 protein levels. USP7 associates with CHK1, leading to its stabilization by deubiquitinylation, and this association is enhanced in response to cytarabine treatment. Pharmacological or RNA interference-mediated inhibition of USP7 significantly reduced AML proliferation in vitro and in vivo, and increased AML cell death. It is important to note that USP7 inhibition synergized with cytarabine to kill AML cell lines. This is also the case in primary patient specimens with high CHK1 levels. Transcriptomic dataset analyses revealed that a USP7 gene signature is highly enriched in cells from AML patients at relapse, as well as in residual blasts from Patient Derived Xenograft (PDX) models treated with clinically relevant doses of cytarabine, strongly suggesting a relationship between USP7 expression and resistance to therapy. Finally, single cell analysis from AML patient at relapse versus diagnosis showed that a gene signature of the pre-existing subpopulation responsible for relapse is enriched in transcriptomes of patients with high USP7 level. Altogether, these data demonstrate that USP7 is a master regulator of CHK1 protein kinase in AML cells, and represents both a marker of resistance to chemotherapeutic treatments, as well as a potential therapeutic target to overcome treatment resistance.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Quinazolinone USP7 inhibitor as well as preparation method and application thereof
ActiveCN112047933AHigh reaction yieldExhibit antitumor activityOrganic chemistryAntineoplastic agentsPharmaceutical drugGastric carcinoma
The invention discloses a quinazolinone USP7 inhibitor, which has a structure shown in a general formula I, has a good inhibition effect on ubiquitin-specific protease 7, can be used for preparing a medicine for inhibiting the ubiquitin-specific protease 7, and shows good inhibition activity on a gastric cancer cell line, especially an MGC803 cell line. A lead compound structure is provided for anti-cancer drugs, and the compound has a good application prospect. The invention further provides a preparation method of the quinazolinone USP7 inhibitor. The preparation method is simple, mild in reaction condition, few in by-product, high in reaction yield and convenient for batch production and commercial application.
Owner:ZHENGZHOU UNIV
4-hydroxypiperidine derivatives and their use as inhibitors of ubiquitin specific protease 19 (USP19)
PendingUS20210070773A1Increased caspase activationLower Level RequirementsOrganic chemistryMetabolism disorderAtrophyPipequaline
Inhibitors of ubiquitin specific protease 19 (USP19) of Formula (I) are provided, together with pharmaceutical compositions comprising said inhibitors, and methods of use thereof. The compounds can be used in in the treatment of muscular atrophy, obesity, insulin resistance or type II diabetes or in reducing the loss of muscle mass.
Owner:ALMAC DISCOVERY LIMITED
Recombinant lentiviral vector containing ubiquitin-specific protease gene USP39-shRNA (short hairpin ribonucleic acid) and application thereof
The invention provides a recombinant lentiviral vector containing ubiquitin-specific protease gene USP39-shRNA (short hairpin ribonucleic acid) and application thereof. The test proves that expression of USP39 in breast cancer tissue is obviously higher than that in normal breast tissue; the immunohistochemical detection confirms that the expression of USP39 in the breast cancer tissue is obviously higher than that in normal breast tissue; the immunohistochemical detection confirms that expression of USP39 protein in the breast cancer tissue is obviously higher than that in para-carcinoma tissue; siRNA (small interfering ribonucleic acid) is designed in a breast cancer cell system to interfere the expression of the USP39; it is found that proliferation of breast cancer cell after reducing the USP39 is obviously restrained; the cell apoptosis ratio is obviously improved; the cell ratio at the G1 stage is increased; the cell ratio at the S sage is reduced; and the clonality of the cell is obviously reduced. The test proves that the USP39 has an important role in facilitation of development of the breast cancer; and theoretical and experimental basis is provided for preparation of a drug for preventing and treating the breast cancer by the lentiviral vector containing USP39-shRNA.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Function and application of ubiquitin-specific protease 4 (usp4) in the treatment of cardiac hypertrophy
ActiveCN105251020BProtect heart functionProtects Against Cardiac FibrosisPeptide/protein ingredientsGenetic material ingredientsCardiac fibrosisCardiac functioning
The invention discloses a function and application of ubiquitin specific protease 4 (USP4) in treating cardiac hypertrophy, and belongs to the field of gene functions and application. The mutual relation between the expression of the USP4 and cardiac hypertrophy is determined; research results show that in a cardiac hypertrophy occurrence model, the expression of the USP4 is significantly reduced compared with a normal group; by restraining the expression of the USP4, the cardiac hypertrophy and fibrosis are significantly promoted, and the cardiac function is deteriorated; by promoting the expression of the USP4, cardiac hypertrophy and fibrosis are significantly restrained, and the cardiac function is protected. Accordingly, the USP4 can be used as a target gene for screening and preparing the medicine which protects the cardiac function, resists cardiac fibrosis and / or prevents, relieves and / or treats cardiac hypertrophy, and an effective new way is provided for treating cardiac hypertrophy.
Owner:武汉惠康基因科技有限公司
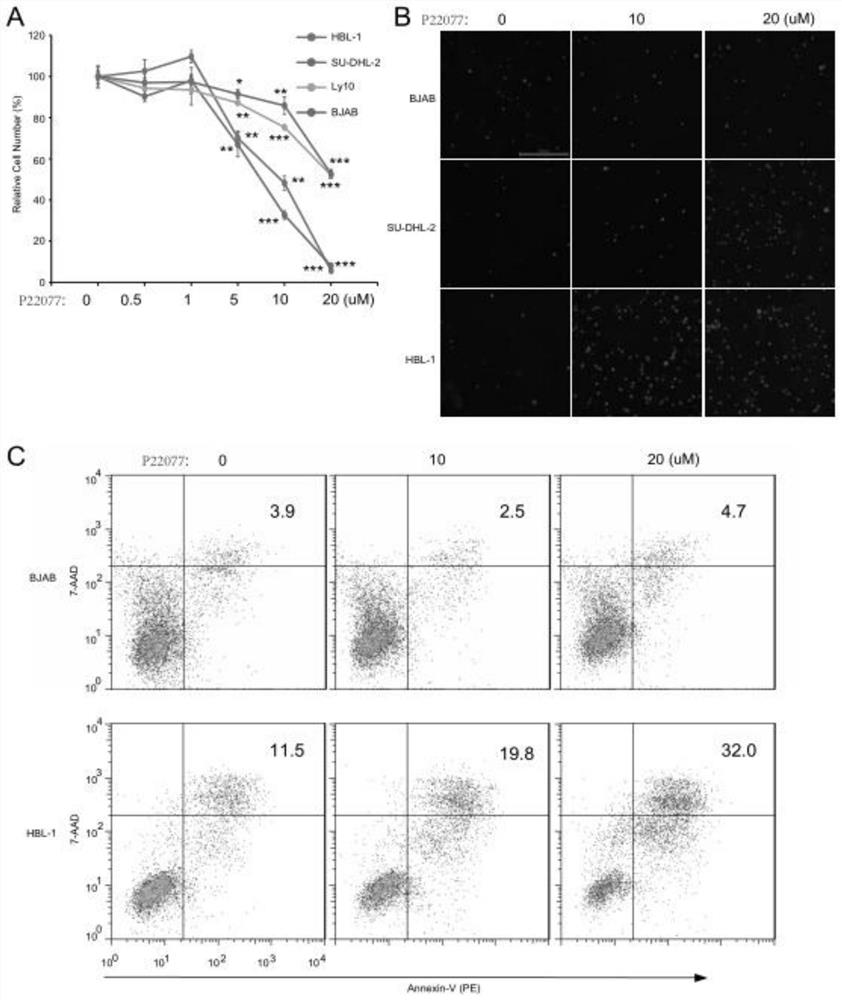
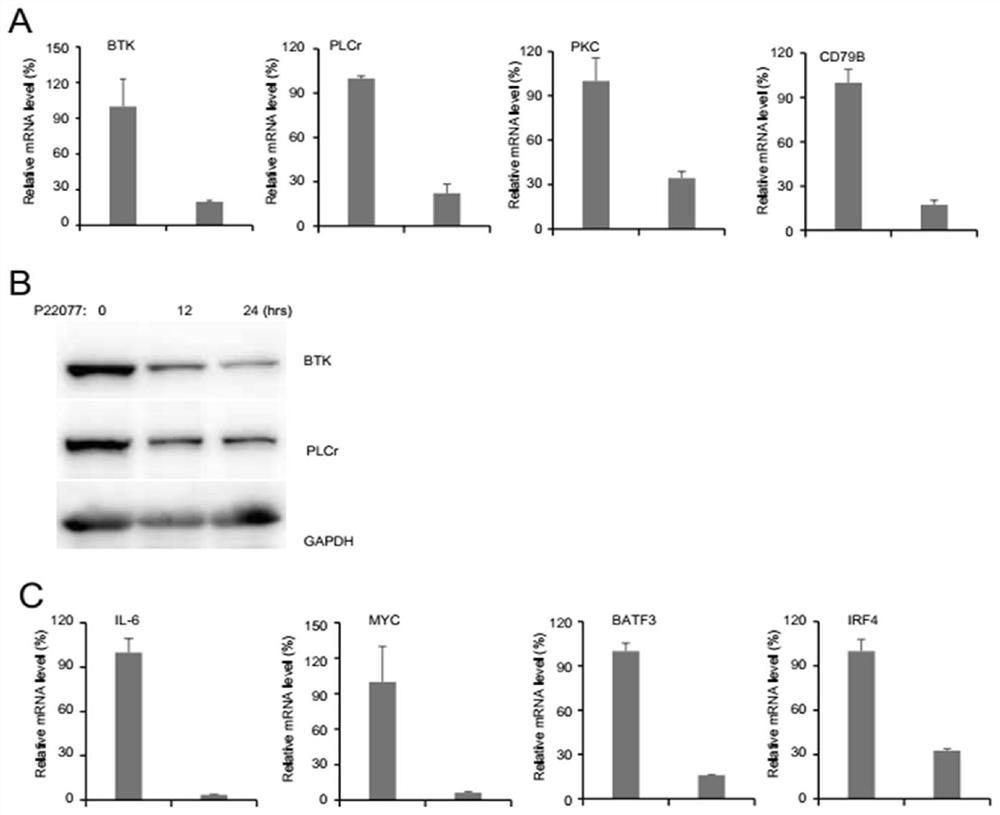
Application of USP7 inhibitor in medicine for treating ABC-DLBCL
InactiveCN111840562AGood application prospectImprove market competitivenessMicrobiological testing/measurementIndividual particle analysisDiseaseStaining
The invention discloses an application of a USP7 inhibitor in a medicine for treating ABC-DLBCL. The expression condition of the USP7 in a DLBCL cell line is studied; through database analysis, a USP7small-molecule inhibitor is used for inhibiting USP7 activity; the influence of ABC type DLBCL cell proliferation, PI staining and flow cytometry on cell apoptosis and the influence of cell apoptosison an ABC type DLBCL BCR signal path by a CCK-8 method can be observed; USP7 is a ubiquitin specific protease which is most widely researched at present; researches show that USP7 participates in regulating the activity and functions of various protein substrates of cells, USP7 expression abnormality or activity change plays an important role in occurrence and development of diseases and tumors,and USP7 has good application prospects and market competitiveness and has a good promotion effect on improvement of people's livelihood and society in China.
Owner:NANTONG UNIVERSITY
A detection, treatment and prognosis target and application of cholangiocarcinoma
ActiveCN110699453BReduced activityPrevent proliferationOrganic active ingredientsHydrolasesNucleotideOncogene
The invention relates to a cholangiocarcinoma cell medicine target point. The medicine target point is ubiquitin specific protease 8, and the nucleotide sequence is shown as SEQID NO:1 in a sequence table. For the first time, the invention puts forward that USP8 is knocked down, so that proliferation, migration and invasion of cholangiocarcinoma cells can be notably restrained, MMP9 activity achieving important effects on cell adhesion, neoplasm invasiveness and transfer is reduced, and the USP8 is promoted to achieve effect of oncogene for growth and transfer ability of cholangiocarcinoma. New possibility is provided for development of cholangiocarcinoma medicines and cholangiocarcinoma prevention, treatment and prognosis.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Method for identifying modulators of G3BP activity
A method of identifying a lead or candidate compound that modulates the activity of GTPase-Activating Protein SH3 Domain-Binding Proteins (G3BP) is provided, which includes determining whether a compound modulates the interaction between the N-terminal Nuclear Transport Factor 2-like (NTF2L) domain of G3BP and FGDF peptide of ubiquitin specific protease 10 (USP10) or non-structural protein 3 (nsP3).
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Compositions and methods for treating cancer
PendingUS20220025349A1High activityHigh expressionOrganic active ingredientsPeptide/protein ingredientsOncologyTumor-infiltrating lymphocytes
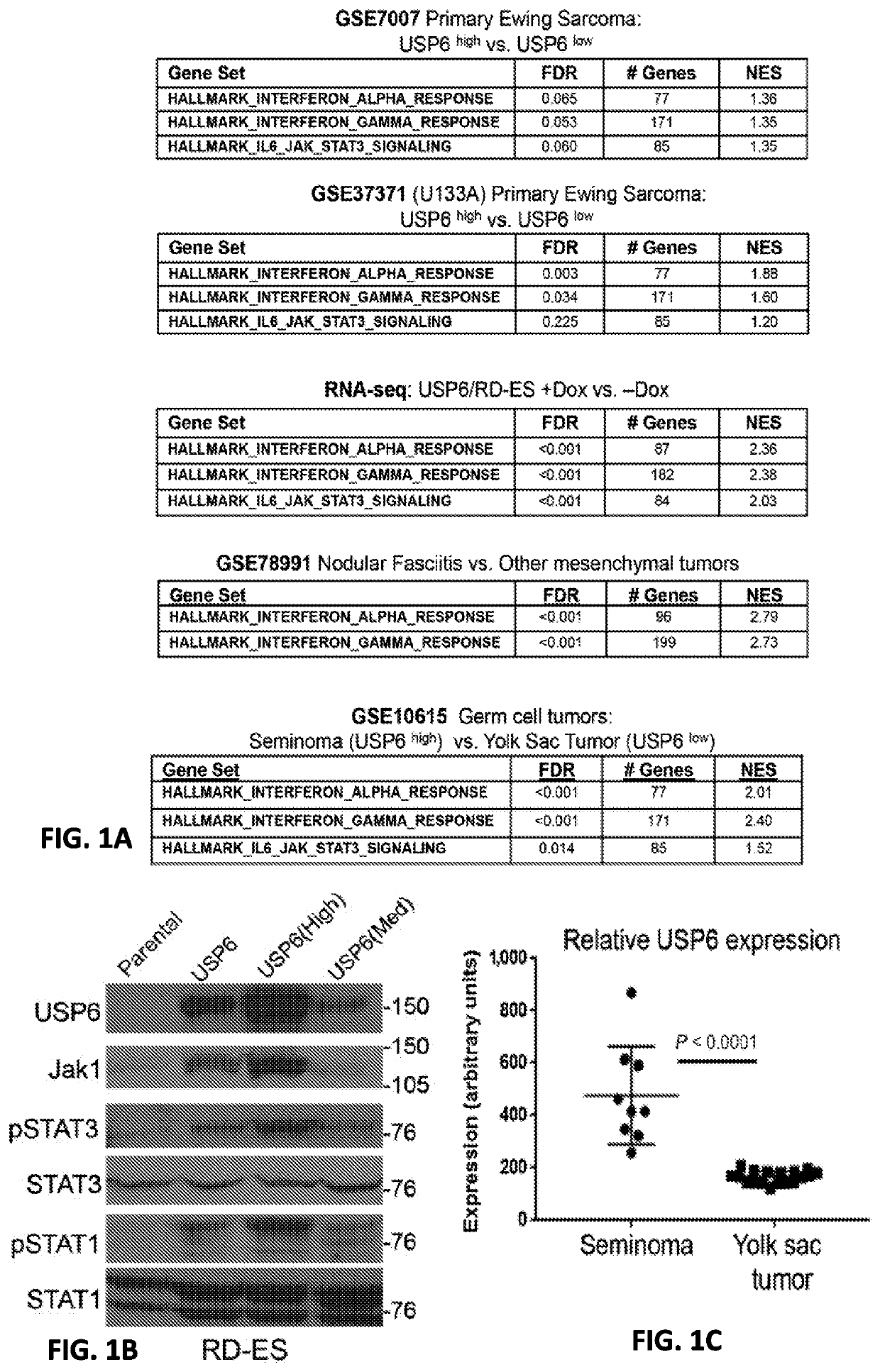
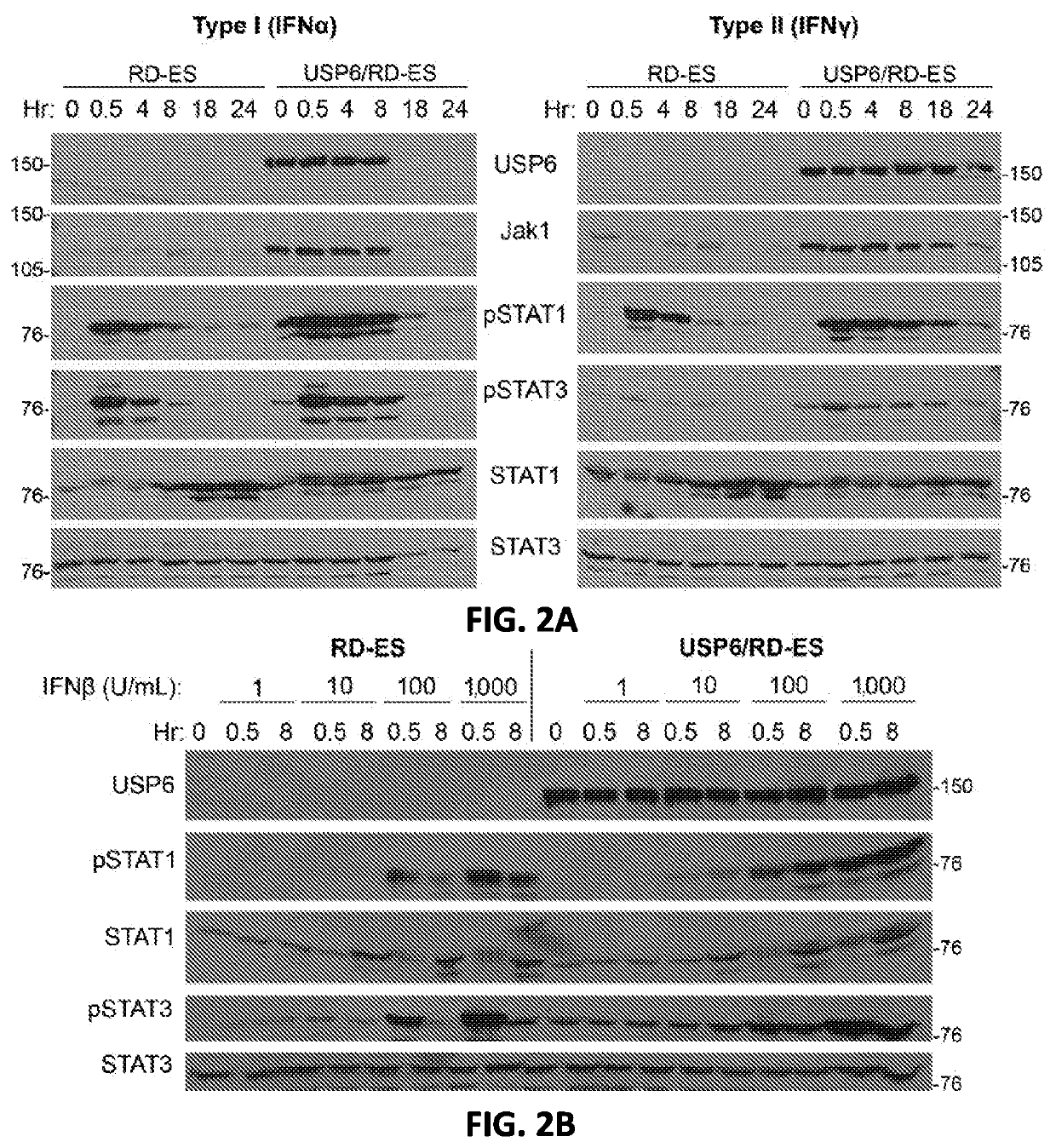
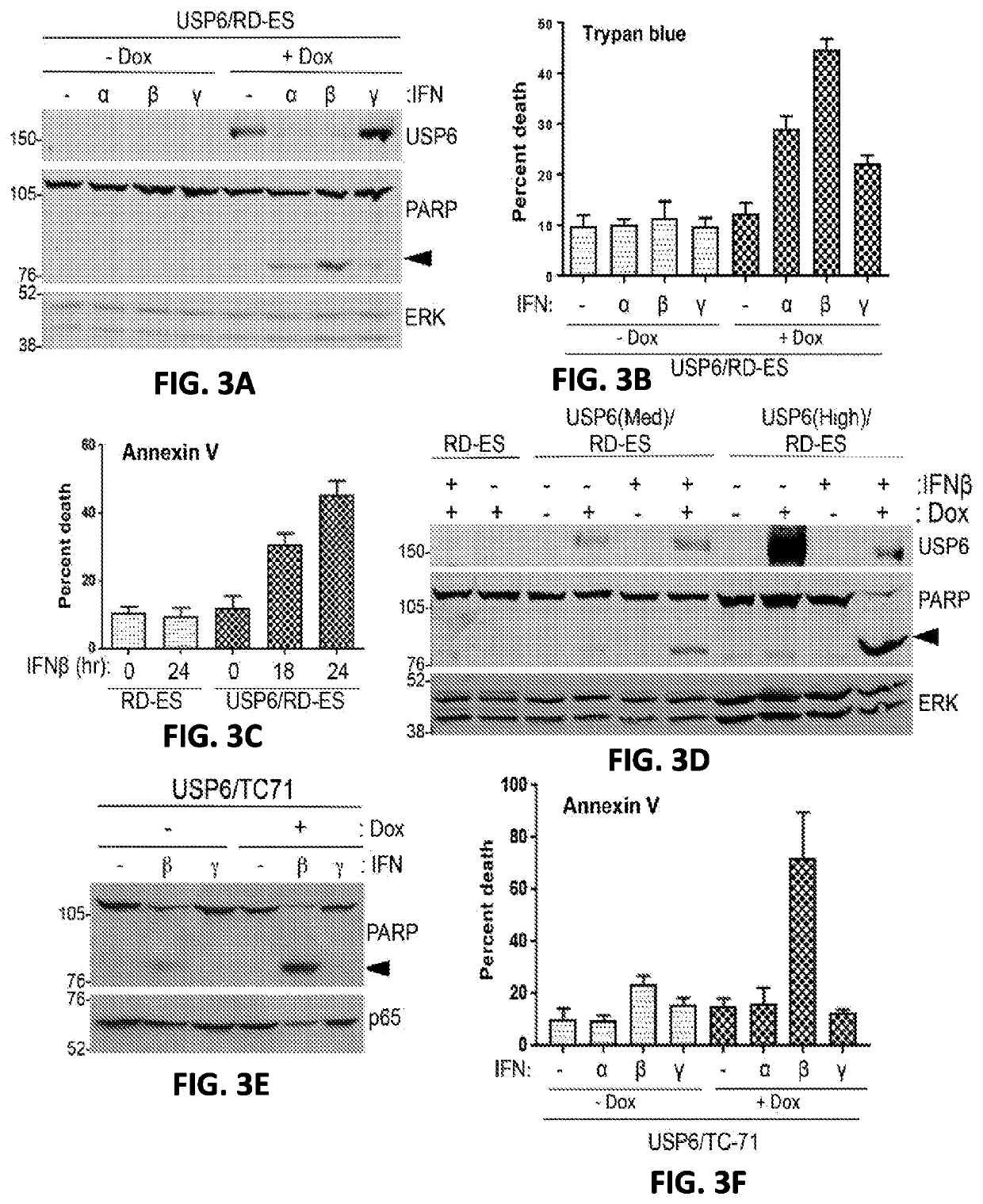
Compositions and methods for treating cancer are provided. In accordance with the instant invention, methods of inhibiting or treating cancer, particularly a “cold” cancer or a cancer lacking CD8+ tumor infiltrating lymphocytes (TILs) and / or other immunostimulatory features, in a subject are provided. In a particular embodiment, the method comprises administering an agent which increases ubiquitin-specific protease 6 (USP6) activity and / or expression to the subject.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use 6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7cd24cf9-8d6b-4bb0-bd2b-f054e70674d2/US20200078336A1-C00001.png)
![6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use 6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7cd24cf9-8d6b-4bb0-bd2b-f054e70674d2/US20200078336A1-C00002.png)
![6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use 6-amino-2,4-dihydropyrano [2,3-c] pyrazoles and methods of use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7cd24cf9-8d6b-4bb0-bd2b-f054e70674d2/US20200078336A1-C00003.png)