Pharmaceutical composition containing USP13 (ubiquitin-specific proteases 13) inhibitor and PARP (poly ADP-ribose polymerase) inhibitor and application thereof
A technology of composition and inhibitor, applied in pharmaceutical composition containing USP13 inhibitor and PARP inhibitor, application field in cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
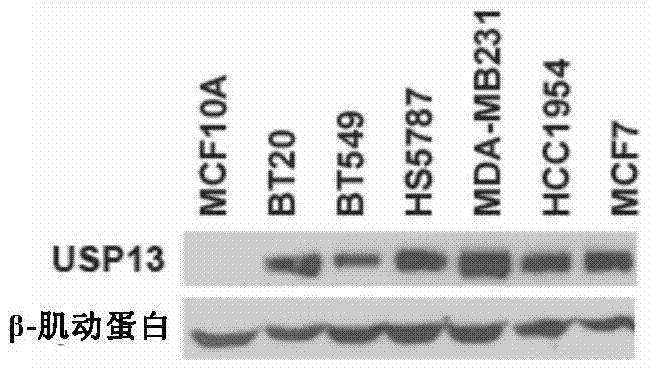
[0143] Expression of deubiquitinating enzyme USP13 in breast cancer cells
[0144] Detection of USP13 expression in MCF10A normal breast cells and BT20, BT549, HS5787, MDA-MB231, HCC1954, MCF7 breast cancer cells
[0145] The result is as figure 1 Shown: The expression of USP13 was not detected in MCF10A normal breast cells, but the expression of USP13 could be detected in BT20, BT549, HS5787, MDA-MB231, HCC1954, and MCF7 breast cancer cells, and the expression of USP13 in MDA-MB231 cells was the highest . Therefore, in the subsequent examples, MDA-MB231 breast cancer cells were selected as the experimental subjects.
Embodiment 2
[0147] Knockout of USP13 using CRISPR-Cas9 system to study the effect on homologous recombination repair efficiency (HR) and cell growth of tumor cell lines
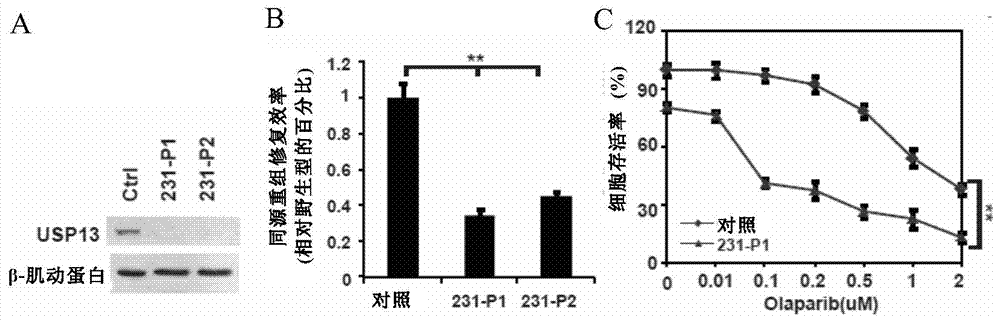
[0148] In MDA-MB231 breast cancer cells, USP13 was knocked out using CRISPR-Cas9 system. Western results showed that the MDA-MB231 cell line that knocked out USP13 through the CRISPR-Cas9 system had been screened to stably deplete USP13, and the two obtained MDA-MB231 cell lines that stably depleted USP13 were named 231-P1 and 231-P2 ( figure 2 A). Homologous recombination repair efficiency (HR) experiments showed that compared with the control, the repair efficiency of MDA-MB231 breast cancer cells 231-P1 and 231-P2 after depleting USP13 was significantly reduced, only 40-50% of the control. ( figure 2 B).
[0149] Olaparib is a commonly used PARP inhibitor. Olaparib can inhibit the DNA damage repair of tumor cells and promote tumor cell apoptosis. In this example, clone formation experiments were performed on th...
Embodiment 3
[0152] Effects of USP13 inhibitors and PARP inhibitors on homologous recombination repair efficiency (HR) and cell growth in breast cancer cell lines
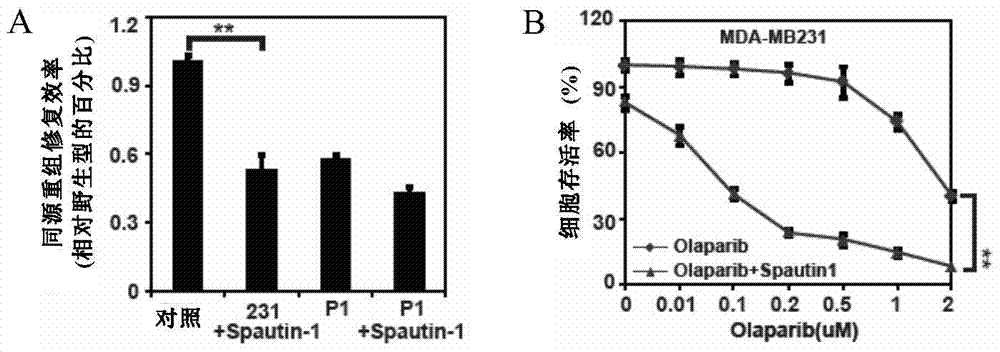
[0153] In this example, the effects of administration of PARP inhibitor Olaparib and USP13 inhibitor Spautin1 on homologous recombination repair efficiency (HR) and cell growth of breast cancer cell lines were studied.
[0154] Homologous recombination repair efficiency experiments are divided into four groups, including:
[0155] Control group: no drug treatment.
[0156] Control cells treated with Spautin1: the concentration of Spautin1 was 1 μM.
[0157] Subtracted USP13 cell group: no drug treatment.
[0158]Depleted USP13 cells administered Spautin1 group: the concentration of Spautin1 was 1 μM.
[0159] The result is as image 3 As shown in A, compared with the control, the use of USP13 inhibitor Spautin1 can significantly reduce the homologous recombination repair efficiency of breast cancer cells, and the repair eff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com