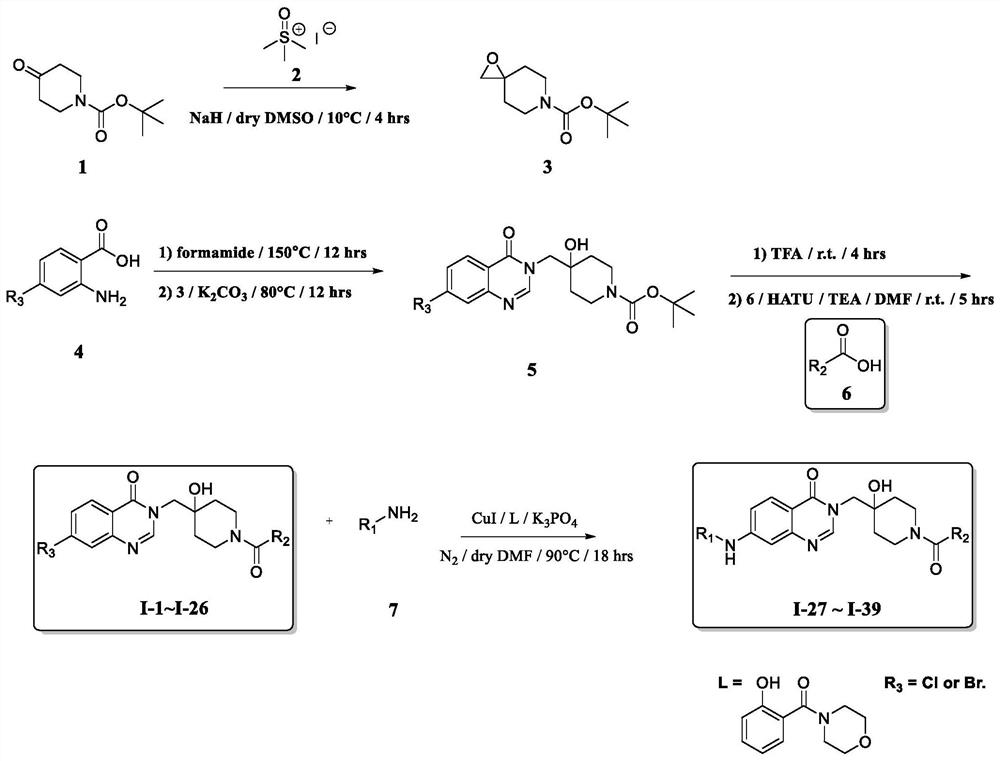
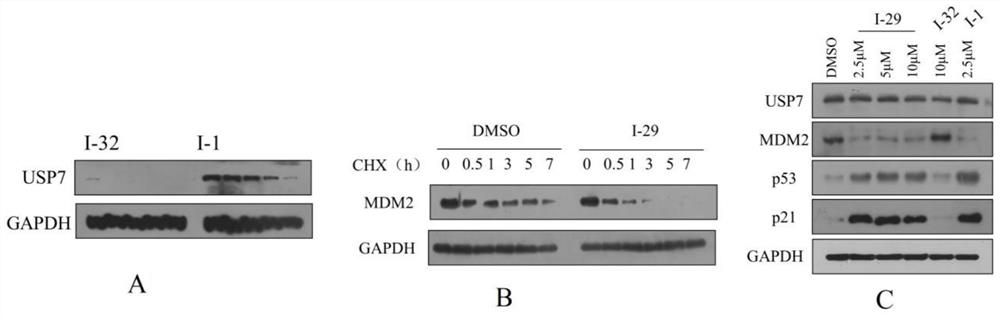
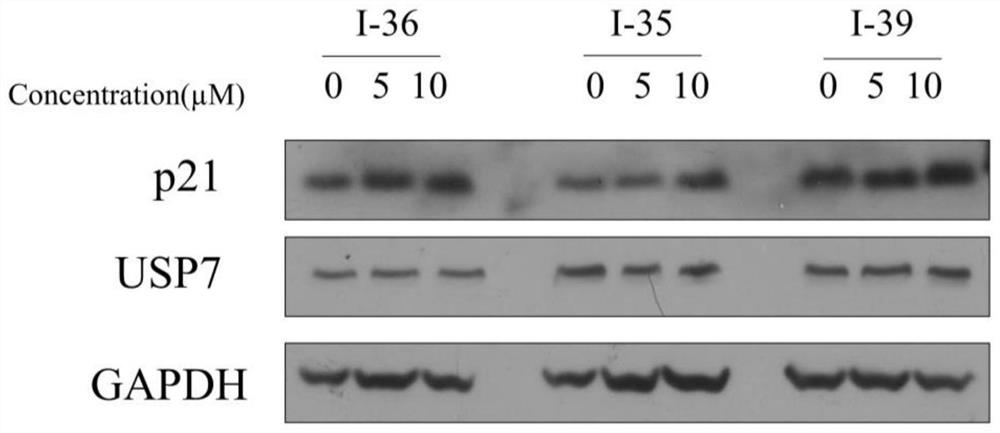
Quinazolinone USP7 inhibitor as well as preparation method and application thereof
A quinazolinone and inhibitor technology, which is applied in the field of quinazolinone USP7 inhibitors and their preparation, can solve problems such as few reports of USP7 inhibitors, and is convenient for mass production and commercial application, and has few by-products. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] compound The synthesis process is as follows:
[0046](1) Synthesis of Compound 3: Dissolve trimethylsulfoxide iodide (3.094g, 14.06mmol, 1.4eqv) in 15mL of anhydrous DMSO, and add sodium hydride (0.562g, 14.06mmol, 1.4eqv, 60% in kerosene), after adding, stir at 0°C for 30 minutes, add N-Boc-4-piperidone (2.0g, 10.04mmol, 1.0eqv), add at this temperature Stirring was continued for 4 hours; 40 mL of water was added, extracted with ethyl acetate (3×90 mL), washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product, which was subjected to column chromatography (PE:EA=10:1, then PE:EA=5:1) to obtain compound 3 (1.285g, white waxy solid, yield 60%). 1 H NMR (400MHz, Chloroform-d) δ 3.71 (dt, J = 9.9, 4.9Hz, 2H), 3.43 (ddd, J = 13.3, 9.5, 3.7Hz, 2H), 2.69 (s, 2H), 1.80 ( ddd,J=13.8,9.4,4.5Hz,2H),1.47(s,11H). 13 C NMR (101MHz, Chloroform-d) δ154.79, 79.73, 77.28, 57.16, 53.74, 42.49, 28.44.
[0047] (2) Synthes...
Embodiment 2
[0051] compound The synthesis process is as follows:
[0052] Wherein the process of step (1) to (3) is with embodiment 1;
[0053] 3-phenylpropionic acid was replaced by benzoic acid, and other operations were the same as in Example 1 to obtain compound I-2 (colorless oil, yield 85%). 1 H NMR (400MHz, DMSO-d 6 )δ8.31(s,1H),8.16(d,J=8.6Hz,1H),7.75(d,J=2.1Hz,1H),7.57(dd,J=8.5,2.1Hz,1H),7.47– 7.42(m,3H),7.39(dd,J=6.7,3.0Hz,2H),5.05(s,1H),4.21(s,1H),4.04(s,2H),3.27(s,1H),3.15 (s,1H),1.63(s,2H),1.54(s,1H),1.36(d,J=13.6Hz,1H). 13 C NMR (101MHz, DMSO-d 6 )δ168.90, 160.18, 150.38, 148.98, 138.87, 136.30, 129.27, 128.41, 128.34, 127.12, 126.62, 126.19, 120.34, 69.40, 53.85. HRMS (ESI): Calcd.C 21 h 20 ClN 3 o 3 ,[M+H] + , m / z: 398.1193, found: 398.1262.
Embodiment 3
[0055] compound The synthesis process is as follows:
[0056] Wherein the process of step (1) to (3) is with embodiment 1;
[0057] 3-phenylpropionic acid was replaced by 2-pyrrole carboxylic acid, and other operations were the same as in Example 1 to obtain compound I-3 (brown solid, yield 45%). Melting point: 116.8-116.9°C. 1 H NMR (400MHz, DMSO-d 6 )δ11.41(s,1H),8.32(s,1H),8.17(d,J=8.6Hz,1H),7.76(d,J=2.2Hz,1H),7.58(dd,J=8.6,2.1 Hz, 1H), 6.87(s, 1H), 6.46(s, 1H), 6.10(q, J=2.8Hz, 1H), 5.04(s, 1H), 4.20–3.94(m, 4H), 3.30(s ,1H),1.72–1.55(m,2H),1.48(d,J=13.4Hz,2H). 13 C NMR (101MHz, DMSO-d 6 )δ161.39,160.18,150.39,148.99,138.89,128.44,127.15,126.21,124.37,120.82,120.34,111.34,108.22,69.50,53.81,34.75. 19 h 19 ClN 4 o 3 ,[M+H] + , m / z: 387.1146, found: 387.1208.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com