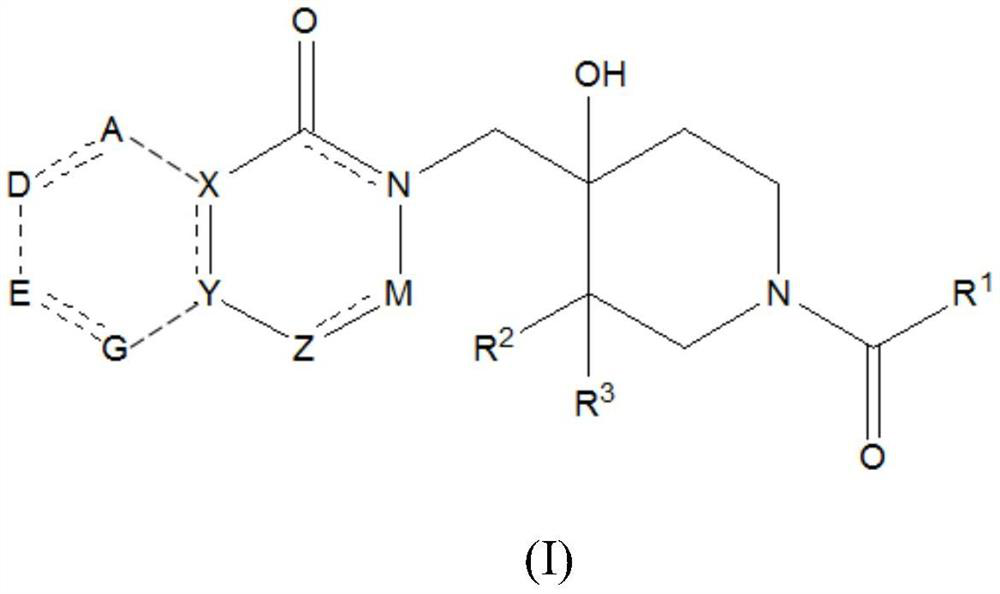
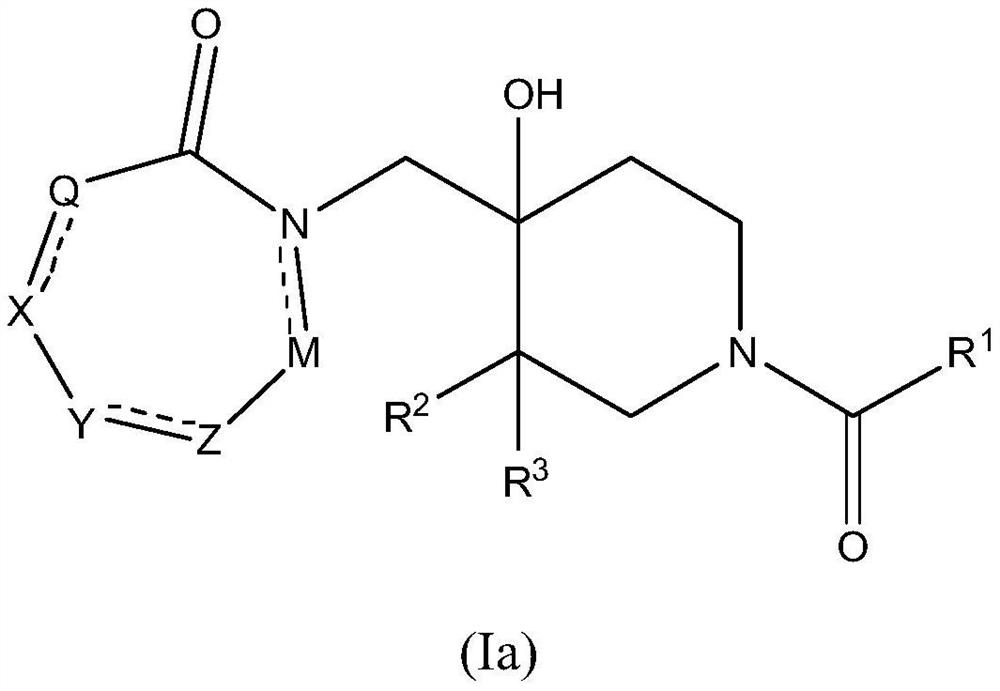
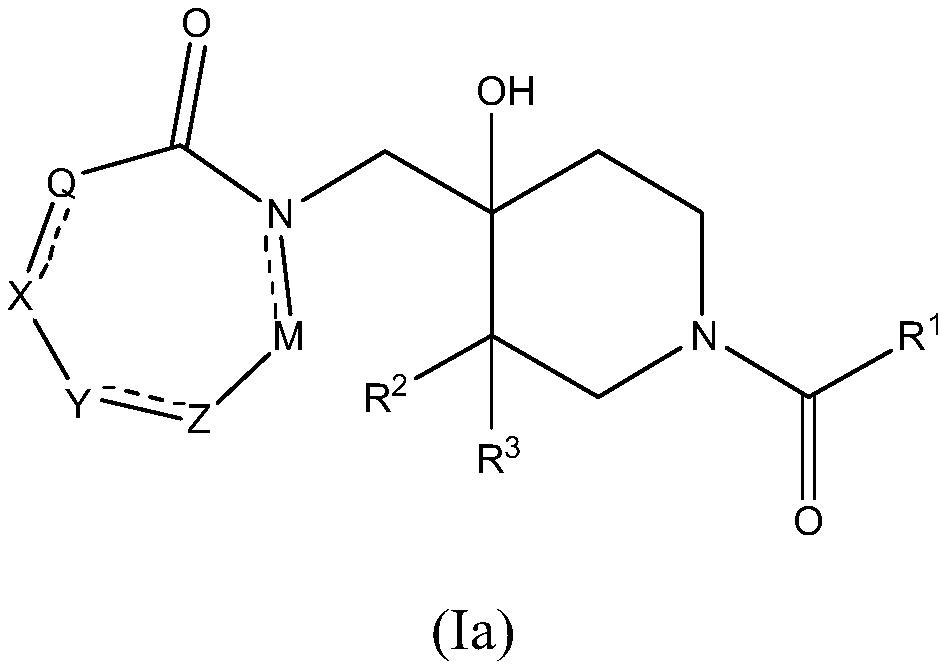
4-hydroxypiperidine derivatives and their use as inhibitors of ubiquitin specific protease 19 (USP19)
A derivative, alkyl technology, applied in the field of inhibitors of ubiquitin-specific protease 19
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0615] For the preparation of solutions and syrups, excipients include, for example, water, polyols, sucrose, invert sugar and dextrose.
[0616] For injection solutions, excipients include, for example, water, alcohols, polyols, glycerin and vegetable oils.
[0617] For suppositories and topical and transdermal administration, excipients include, for example, natural or hardened oils, waxes, fats and semi-solid or liquid polyols.
[0618] The pharmaceutical composition may also contain preservatives, solubilizers, stabilizers, wetting agents, emulsifiers, sweeteners, colorants, flavor enhancers, buffers, coating agents and / or antioxidants.
[0619] For combination therapy, the second drug may be provided in the pharmaceutical composition of the invention, or may be provided alone.
[0620] Pharmaceutical preparations for oral administration can thus be, for example, granules, tablets, dragees, capsules, pills, suspensions or emulsions. For parenteral injections for eg intra...
Embodiment 1
[0783] Example 1: 1-((1-((R)-3-cyclohexyl-2-methylpropionyl)-4-hydroxyl-3,3-dimethylpiperidin-4-yl) Methyl)pyrazin-2(1H)-one
[0784]
[0785] Step 1: tert-butyl 4-hydroxy-3,3-dimethyl-4-((2-oxopyrazin-1(2H)-yl)methyl)piperidine-1-carboxylate: follow general procedure 2 , using pyrazin-2(1H)-one (30 mg, 0.312 mmol), epoxide 1 (98 mg, 0.406 mmol) and cesium carbonate (204 mg, 0.624 mmol) in NMP (1 mL), heated to 80 °C, Hold for 3 hours for preparation to afford the title compound (50 mg, 47%). LCMS (Method A): R T =1.15min, m / z=338[M+H] + ;282[M-Butene+H] + .
[0786] Step 2: 1-((4-Hydroxy-3,3-dimethylpiperidin-4-yl)methyl)pyrazin-2(1H)-one: Follow general procedure 3 using 4-hydroxy-3, 3-Dimethyl-4-((2-oxopyrazin-1(2H)-yl)methyl)piperidine-1-carboxylic acid tert-butyl ester (50 mg, 0.148 mmol), DCM (1 mL) and TFA ( 0.5 mL), stirred at room temperature for 2 hours to obtain the title compound (35 mg, quantitative). LCMS (Method A): R T =0.37min, m / z=238[M+H] + ....
Embodiment 2
[0788] Example 2: 1-((1-((R)-3-cyclohexyl-2-methylpropionyl)-4-hydroxy-3,3-dimethylpiperidin-4-yl) Methyl)-5-phenylpyrazin-2(1H)-one
[0789]
[0790] Step 1: tert-butyl 4-((5-bromo-2-oxopyrazin-1(2H)-yl)methyl)-4-hydroxy-3,3-dimethylpiperidine-1-carboxylate: Following general procedure 2 using 5-bromopyrazin-2(1H)-one (2.62 g, 15 mmol), epoxide 1 (3.98 g, 16.5 mmol) and DIPEA (13.1 mL, 75 mmol) in NMP (30 mL) , heated to 110°C for 20 hours to obtain the title compound (850 mg, 13%). LCMS (Method B): R T =1.18min, m / z=316,318[M-Boc+H] + .
[0791]Step 2: 5-Bromo-1-((4-hydroxy-3,3-dimethylpiperidin-4-yl)methyl)pyrazin-2(1H)-one: Follow general procedure 3 using 4- ((5-Bromo-2-oxopyrazin-1(2H)-yl)methyl)-4-hydroxy-3,3-dimethylpiperidine-1-carboxylic acid tert-butyl ester (850mg, 2.04mmol) , DCM (10 mL) and TFA (5 mL) were prepared by stirring at room temperature for 30 min to afford the title compound (510 mg, 79%). LCMS (Method B): R T =0.41min, m / z=316,318[M+H] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com