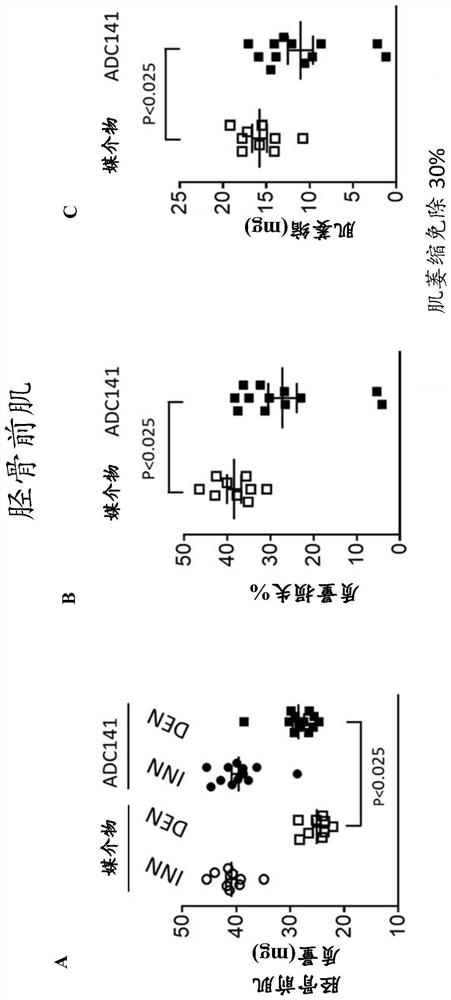
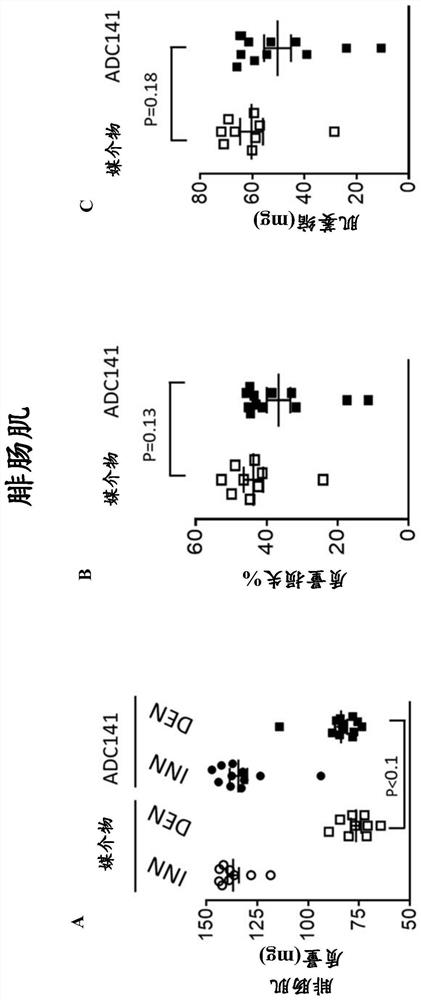
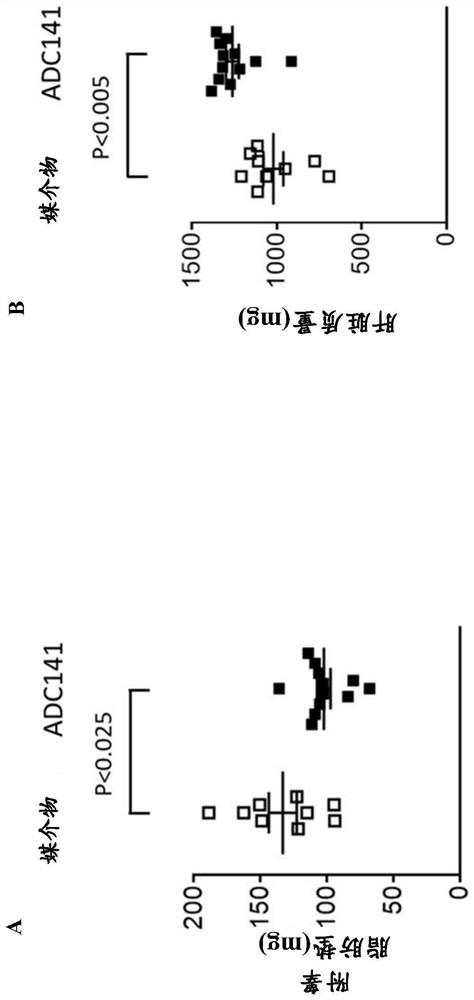
Pharmaceutical compounds and their use as inhibitors of ubiquitin specific protease 19 (USP19)
A compound and hydrate technology, applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0722] For the preparation of tablets, coated tablets or hard gelatine capsules, the compounds of the invention can be mixed with pharmaceutically inert, inorganic or organic excipients. Examples of suitable excipients include lactose, corn starch or derivatives thereof, talc or stearic acid or salts thereof. Suitable excipients for soft gelatine capsules include, for example, vegetable oils, waxes, fats and semi-solid or liquid polyols.
[0723] For the preparation of solutions and syrups, excipients include, for example, water, polyols, sucrose, invert sugar and dextrose.
[0724] For injectable solutions, excipients include, for example, water, alcohols, polyols, glycerol and vegetable oils.
[0725] For suppositories and topical and transdermal applications, excipients include, for example, natural or hardened oils, waxes, fats and semi-solid or liquid polyols.
[0726] The pharmaceutical composition may also contain preservatives, solubilizers, stabilizers, wetting agen...
Embodiment 1
[0949] Example 1: 1-((1-((R)-3-cyclohexyl-2-methylpropionyl)-4-hydroxyl-3,3-dimethylpiperidin-4-yl) Methyl)pyrazin-2(1H)-one
[0950]
[0951] Step 1: tert-butyl 4-hydroxy-3,3-dimethyl-4-((2-oxopyrazin-1(2H)-yl)methyl)piperidine-1-carboxylate: according to general procedure 2 , using pyrazin-2(1H)-one (30 mg, 0.312 mmol), epoxide 1 (98 mg, 0.406 mmol) and cesium carbonate (204 mg, 0.624 mmol) in NMP (1 mL) (heated to 80 °C for 3h) Preparation to give the title compound (50 mg, 47%). LCMS (Method A): R T =1.15min, m / z=338[M+H] + ;282[M-Butene+H] + .
[0952] Step 2: 1-((4-Hydroxy-3,3-dimethylpiperidin-4-yl)methyl)pyrazin-2(1H)-one: According to general procedure 3, using 4-hydroxy-3, 3-Dimethyl-4-((2-oxopyrazin-1(2H)-yl)methyl)piperidine-1-carboxylic acid tert-butyl ester (50 mg, 0.148 mmol), DCM (1 mL) and TFA ( 0.5 mL) (stirred at rt for 2 h) to afford the title compound (35 mg, quantitative). LCMS (Method A): R T =0.37min, m / z=238[M+H] + .
[0953] Step 3: 1...
Embodiment 2
[0954] Example 2: 1-((1-((R)-3-cyclohexyl-2-methylpropionyl)-4-hydroxy-3,3-dimethylpiperidin-4-yl) Methyl)-5-phenylpyrazin-2(1H)-one
[0955]
[0956] Step 1: tert-butyl 4-((5-bromo-2-oxopyrazin-1(2H)-yl)methyl)-4-hydroxy-3,3-dimethylpiperidine-1-carboxylate: According to general procedure 2, using 5-bromopyrazin-2(1H)-one (2.62 g, 15 mmol), epoxide 1 (3.98 g, 16.5 mmol) and DIPEA (13.1 mL, 75 mmol) in NMP (30 mL) ) (heated to 110°C for 20h) to obtain the title compound (850mg, 13%). LCMS (Method B): R T =1.18min, m / z=316,318[M-Boc+H] + .
[0957]Step 2: 5-Bromo-1-((4-hydroxy-3,3-dimethylpiperidin-4-yl)methyl)pyrazin-2(1H)-one: According to general procedure 3, using 4- ((5-Bromo-2-oxopyrazin-1(2H)-yl)methyl)-4-hydroxy-3,3-dimethylpiperidine-1-carboxylic acid tert-butyl ester (850mg, 2.04mmol) , DCM (10 mL) and TFA (5 mL) (stirring at rt for 30 min) gave the title compound (510 mg, 79%). LCMS (Method B): R T =0.41min, m / z=316,318[M+H] + .
[0958] Step 3: 5-Bro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com