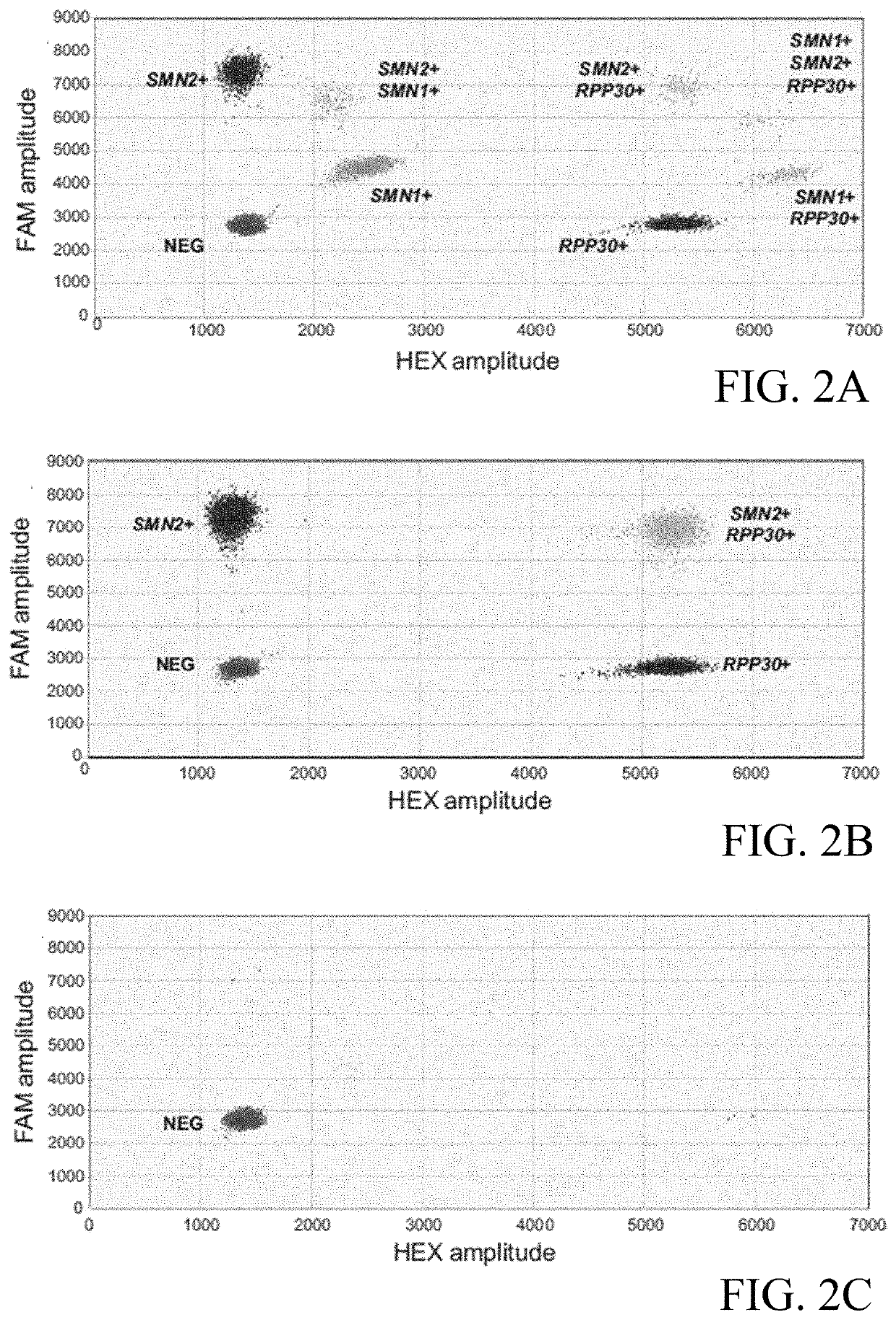
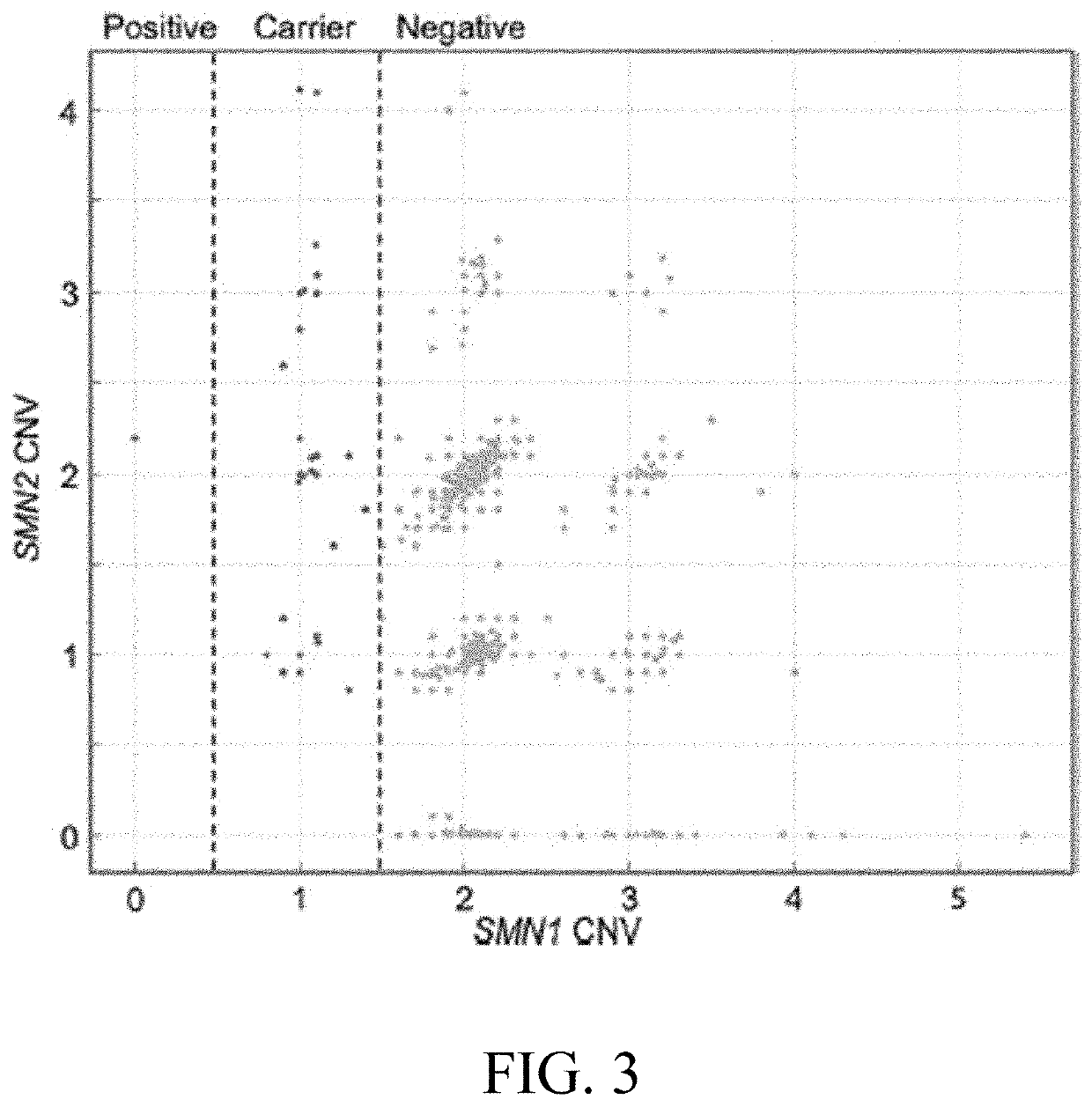
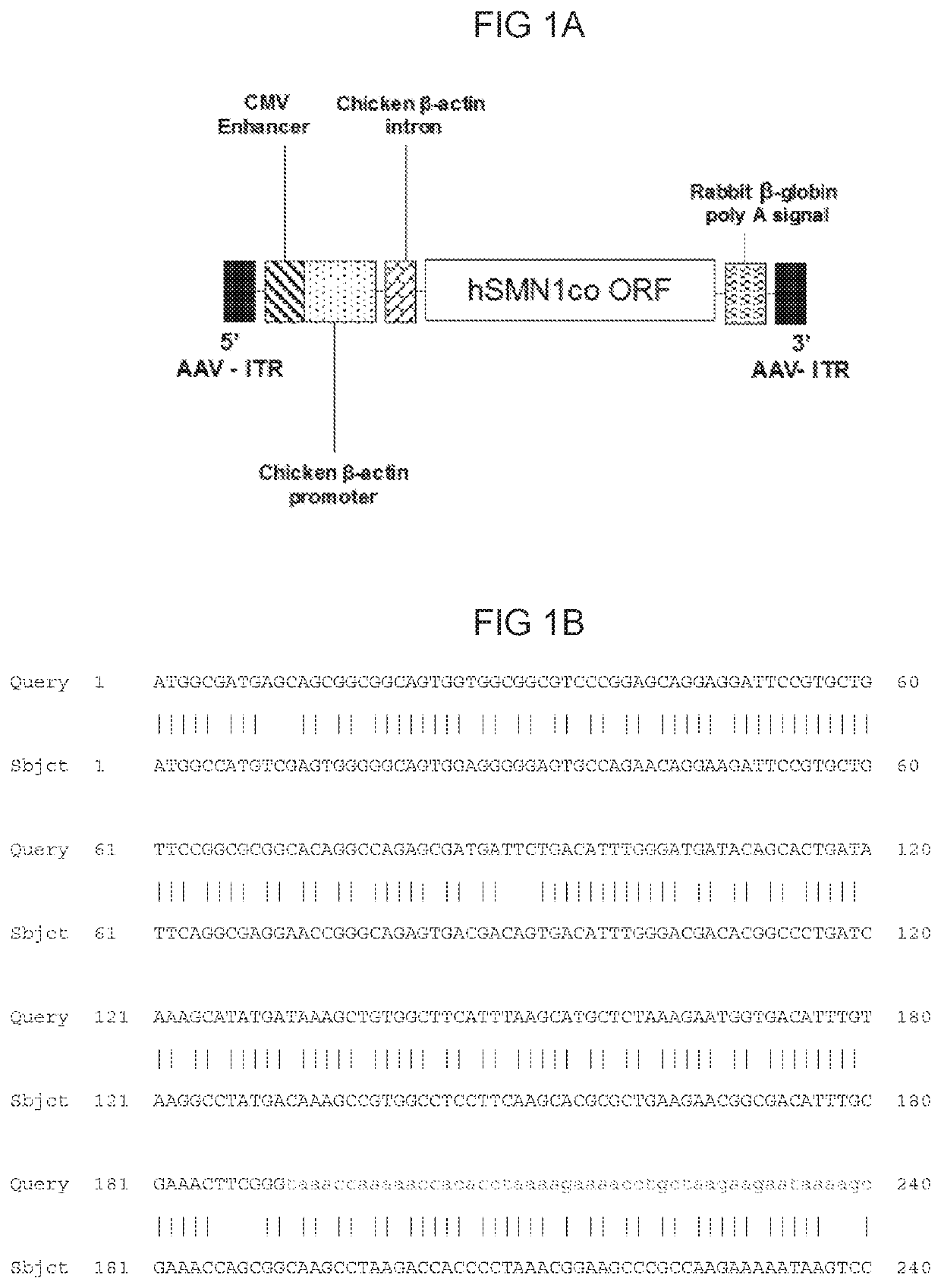
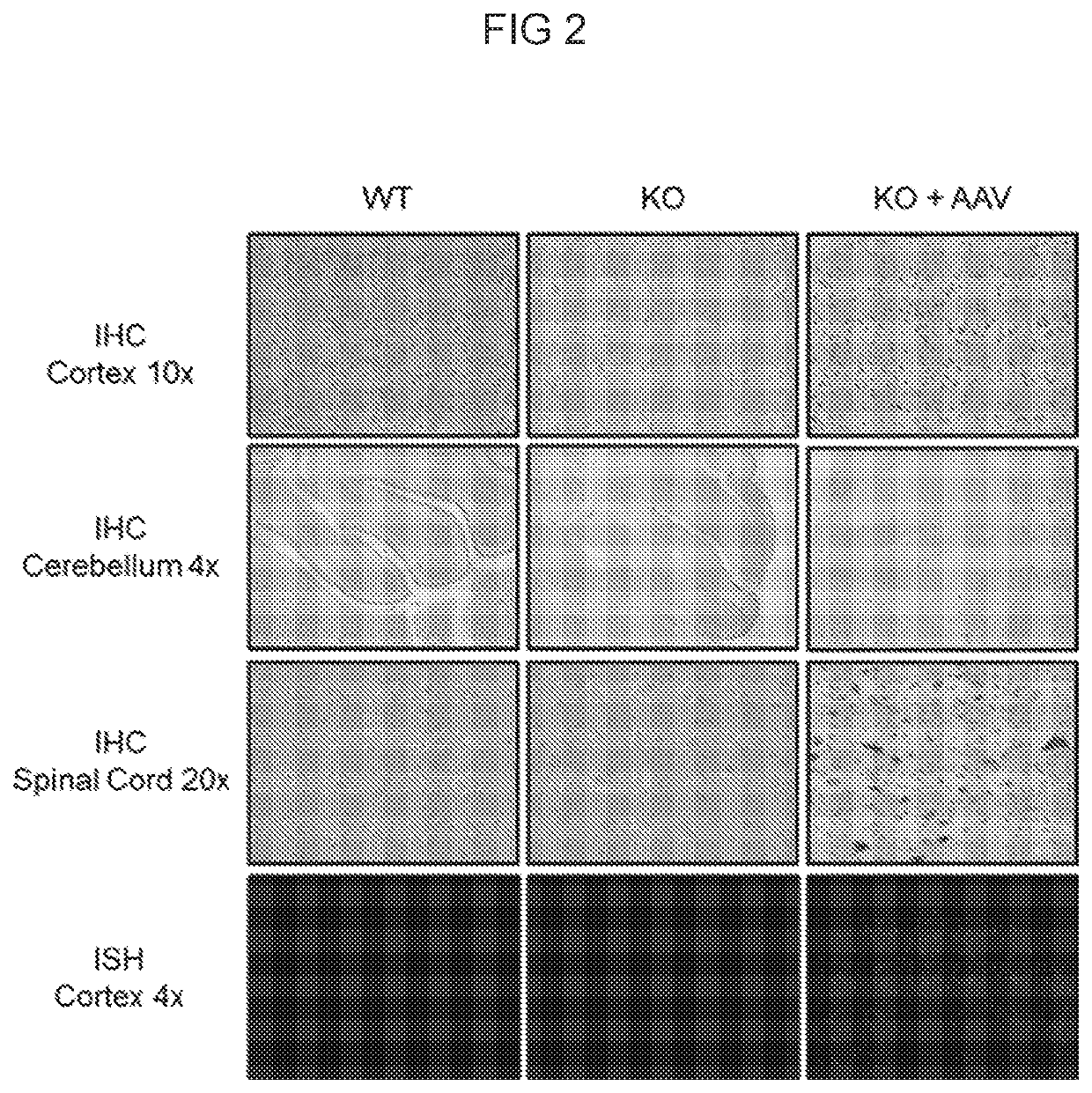
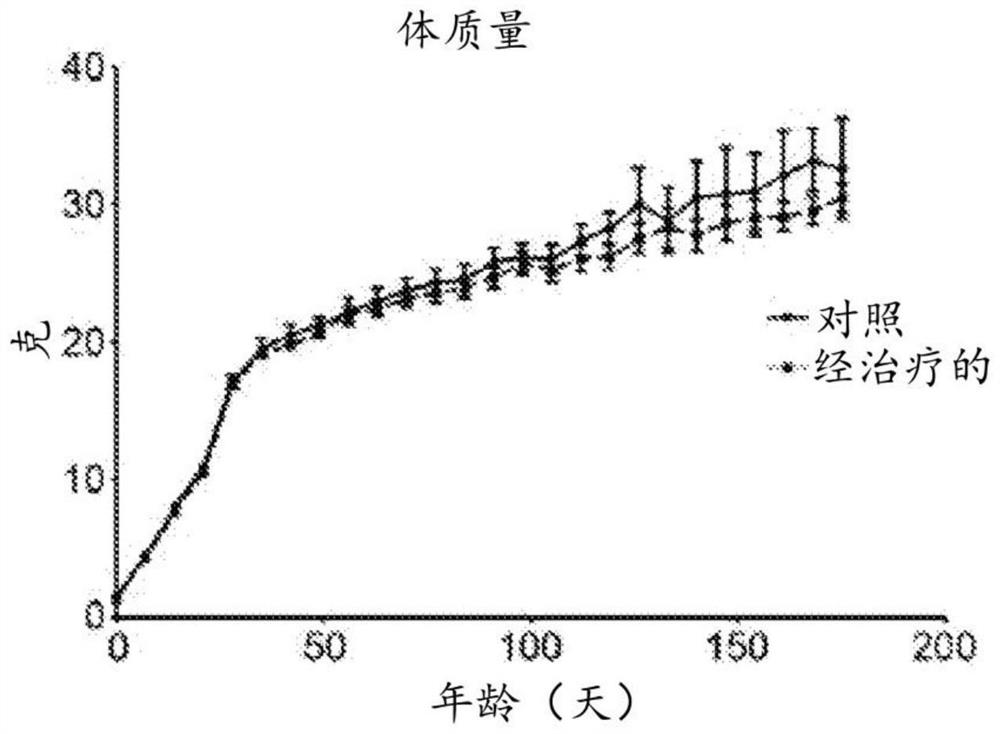
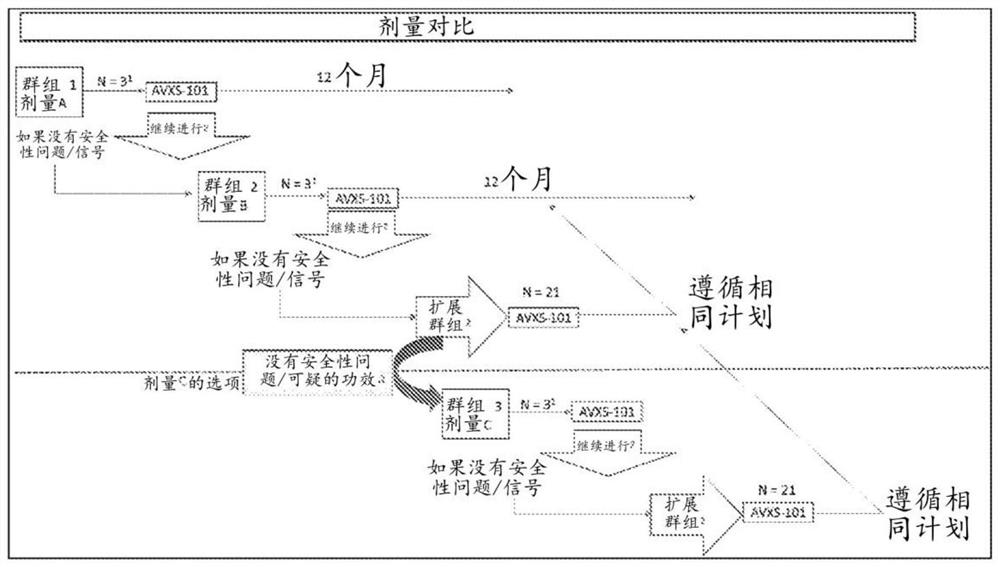
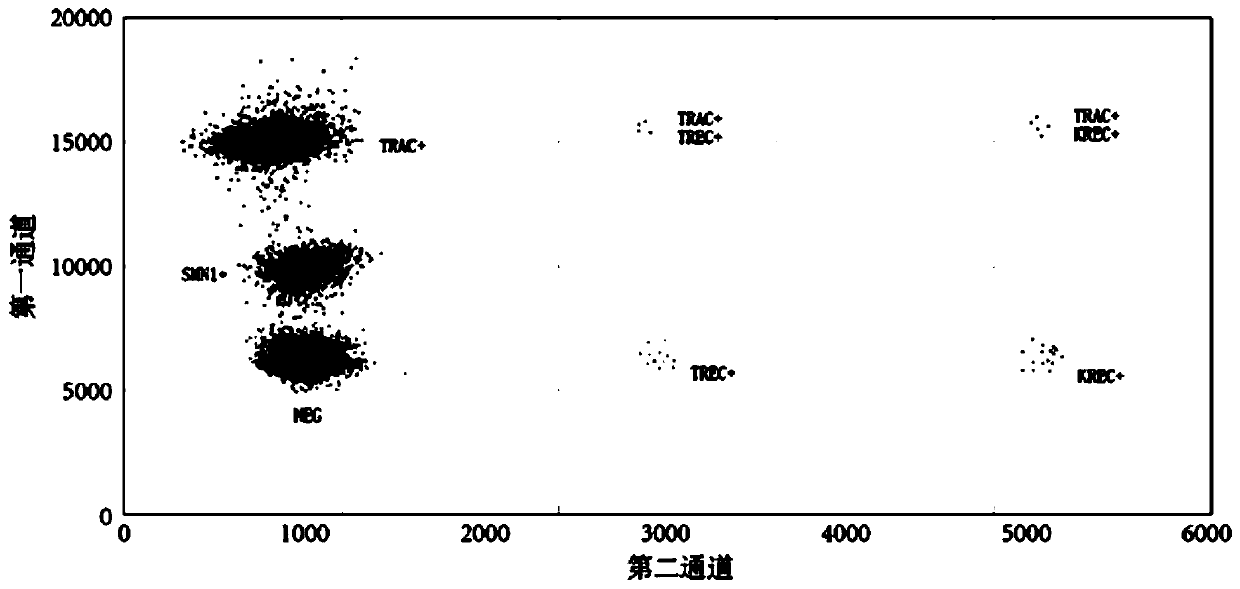
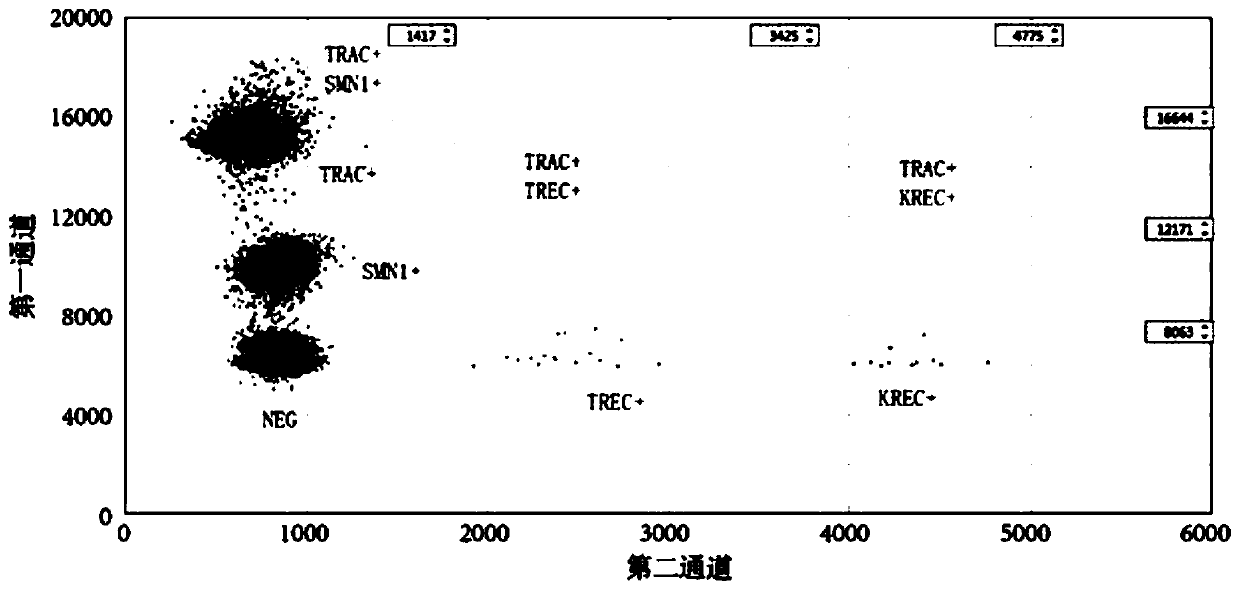
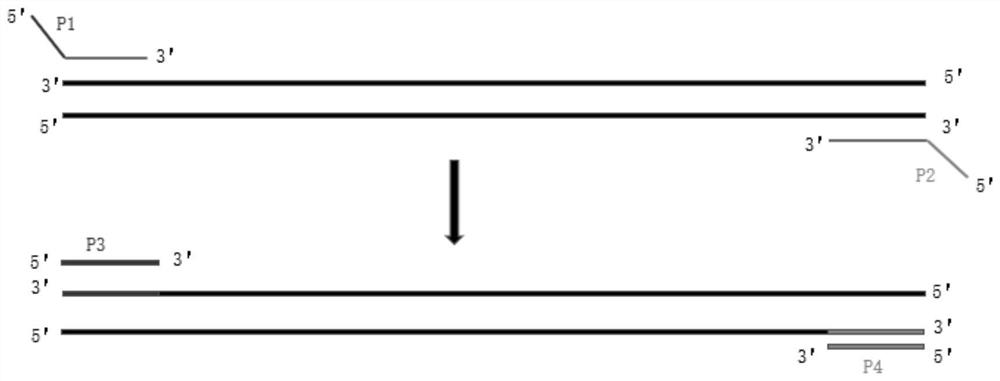
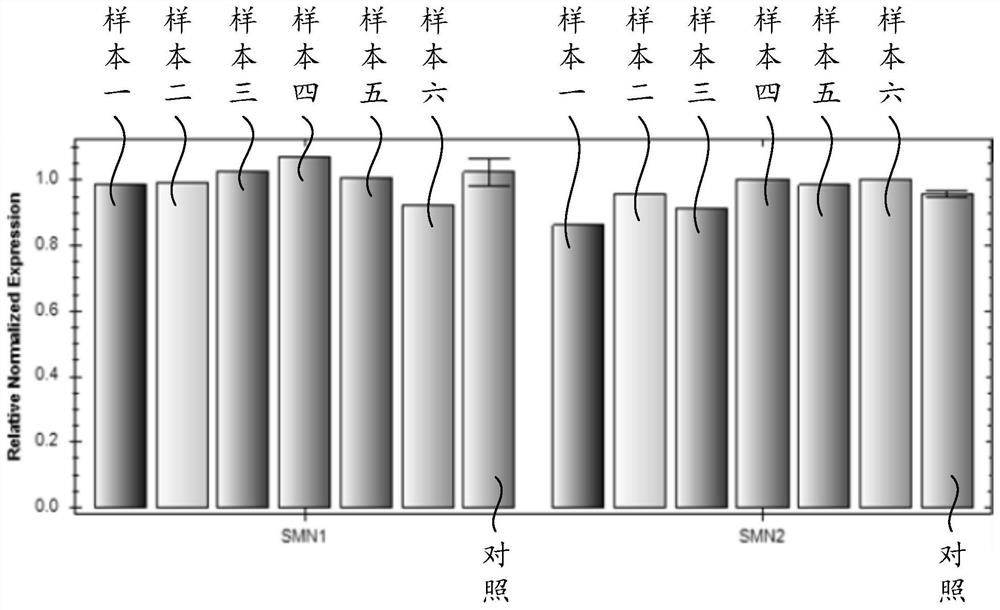
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Muscular spinal atrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
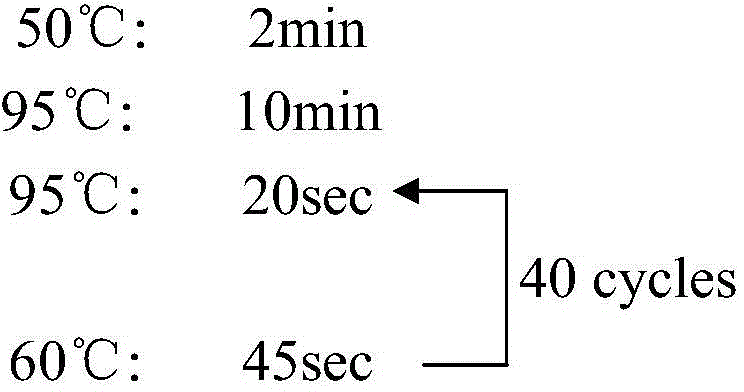
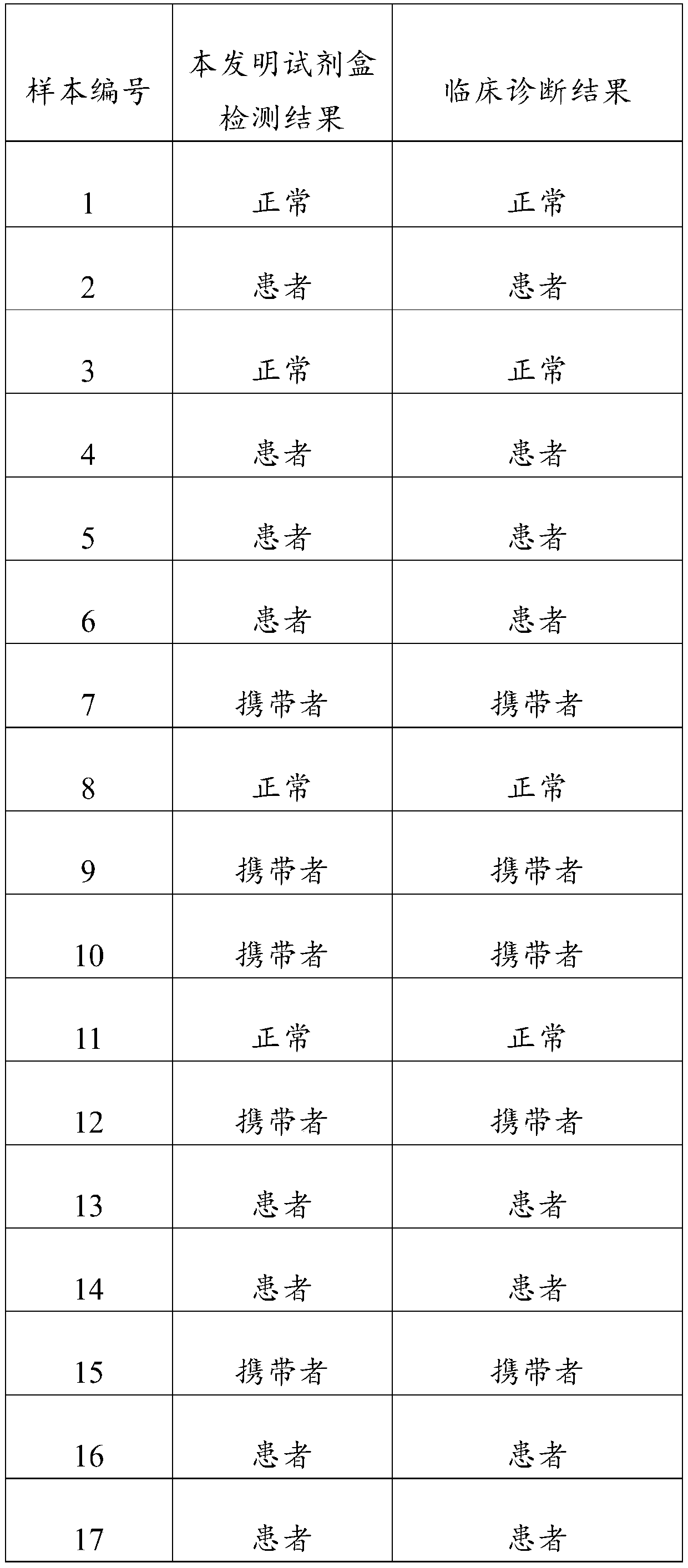
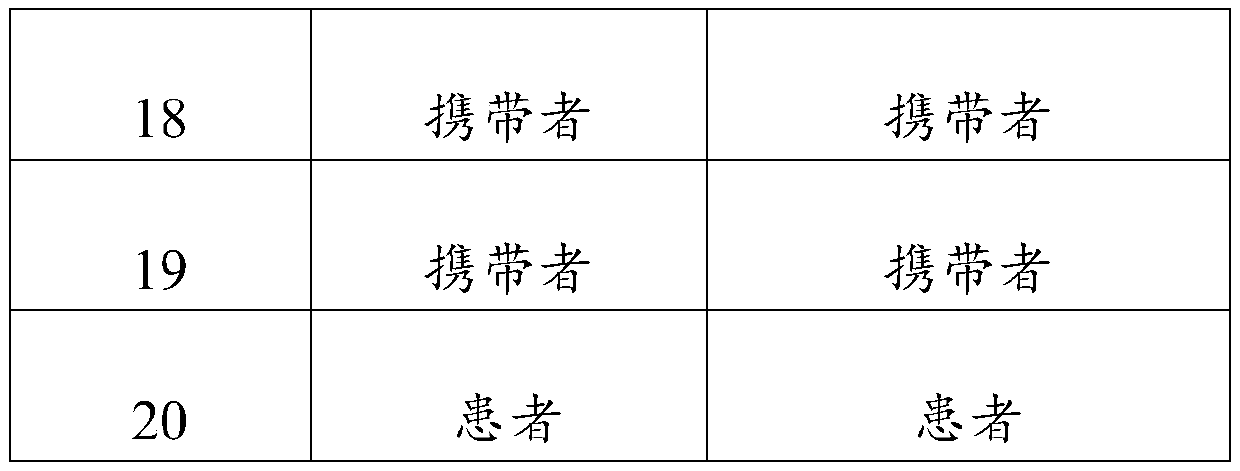
Primer, probe and kit for fluorescent quantitative detection on genes of spinal muscular atrophy (SMA)
ActiveCN104480206AEasy to detectAvoid false negativesMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceSmn gene
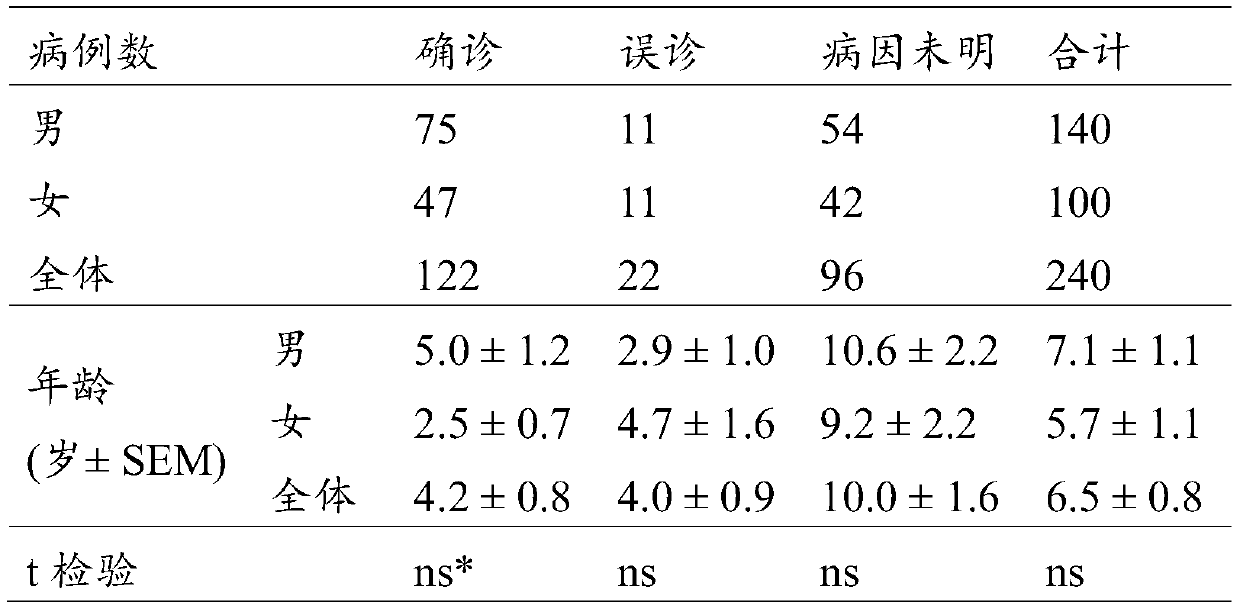
The invention relates to the field of gene detection, and particularly relates to a primer, a probe and a kit for fluorescent quantitative detection on genes of spinal muscular atrophy (SMA). The kit comprises the primer and the probe for fluorescent quantitative detection on genes of SMA, wherein the primer includes 9 pairs of primers; the probe includes 15 probes. According to the primer, the probe and the kit for fluorescent quantitative detection on genes of SMA, a fluorescent quantitative PCR technology is utilized, common mutation site detection which is not found in existing patent technologies is added, so that, 16 types of SMA patients caused by SMN gene deletion, transformation and site mutation can be specifically detected in one time, and classification of SMA patients can be realized.
Owner:亚能生物技术(深圳)有限公司
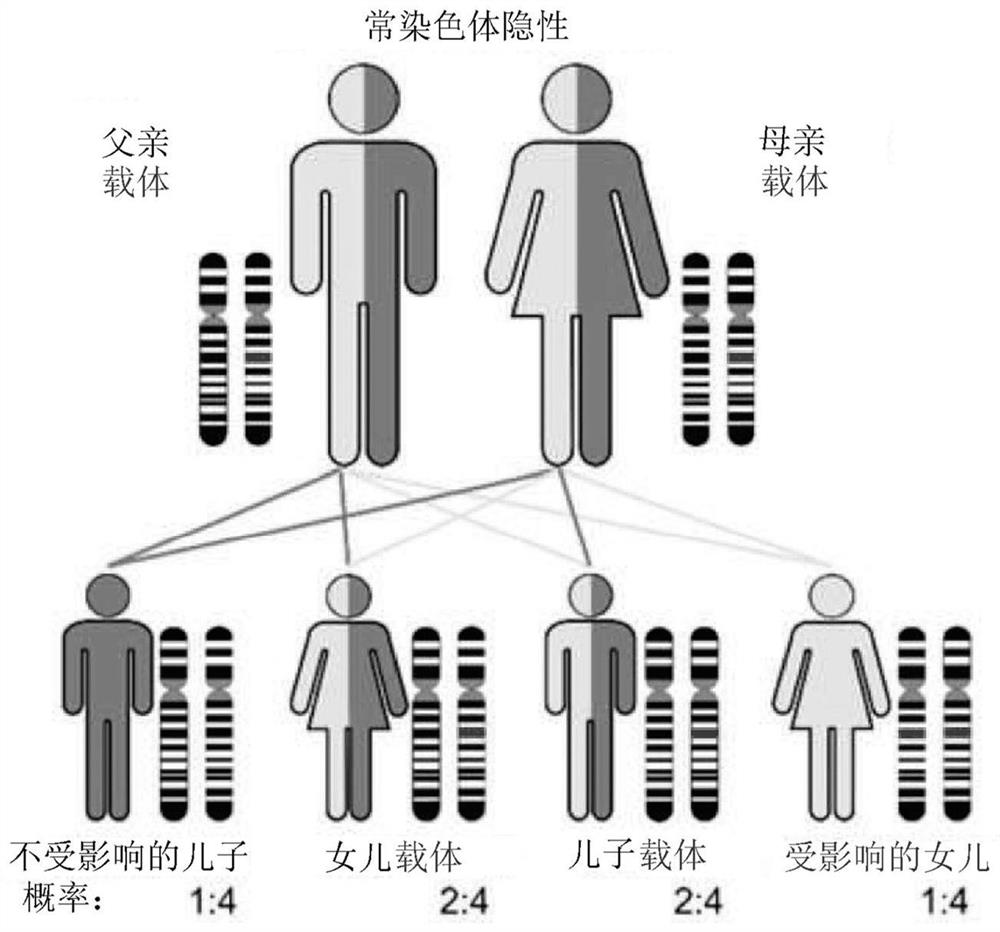
Utilization of nucleotide probes for the measurement of specific mRNA for the molecular diagnosis of autosomal recessive spinal muscular atrophy
InactiveUS20030049627A1Sugar derivativesMicrobiological testing/measurementSingle-strand conformation polymorphismNucleotide
The present invention concerns the development of a quantitative method for the molecular diagnosis of autosomal recessive spinal muscular atrophy (SMA) by measuring the amount of cytosolic mRNA from human muscle cells. Both the procedure using radioactive material and the Enzyme-Linked Immunosorbent Assay (ELISA) nonradioactive method were developed using 32P-dCTP labeled and biotinylated nucleotide probes. The results obtained demonstrate that the measurement of mRNA could be used as a quantitative method for the molecular diagnosis of SMA. There was a perfect concordance of the results obtained between the procedure using radioactive material, the ELISA method and the single strand conformation polymorphism (SSCP) analysis regarding the negative and positive SMA samples. The methods developed in this study may be applicable to the diagnosis (detection of homozygous and heterozygous deletions in exons 7 and 8 of the SMN gene) and the control of mRNA concentrations in the future gene therapy of patients with SMA.
Owner:NGUYEN HUYNH MAI THI
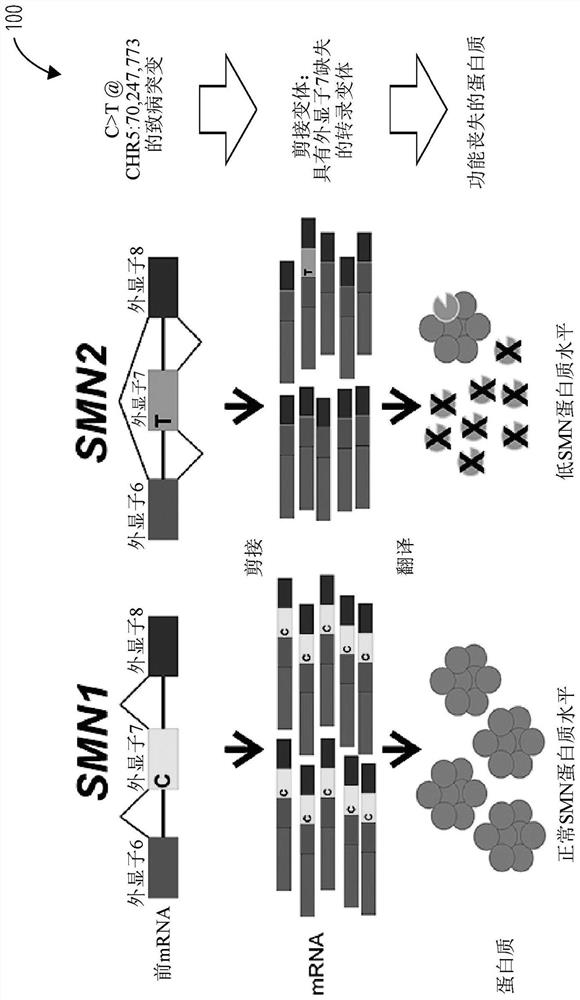
Methods for treating spinal muscular atrophy
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a subject. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy. Also provided are kits for detecting the amount of SMN protein in a sample of cerebrospinal fluid.
Owner:BIOGEN MA INC
Method for detecting spinal muscular atrophy virulence gene
InactiveCN104762398ASequencing implementationExact copy numberMicrobiological testing/measurementSpinal cordInsertion
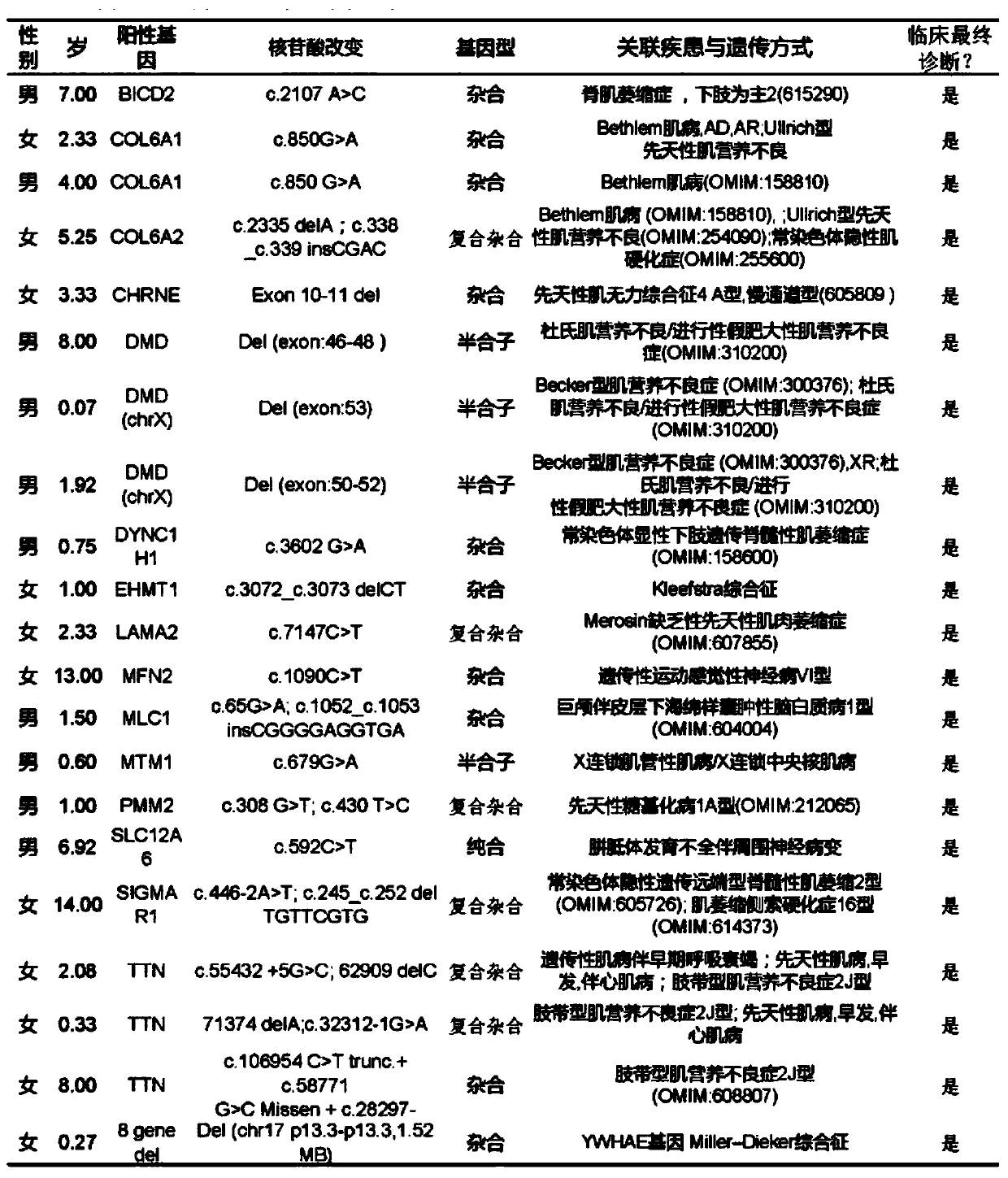
The invention relates to a method for detecting a genetic disease gene and particularly discloses a method for detecting a spinal muscular atrophy virulence gene. The method comprises the following steps: 1, performing enrichment processing on SMN1 and SMN2; 2, preparing a nanopore sequencing library for sequencing; 3, sequencing by a sequenator; and 4, performing clinical report evaluation on the obtained sequence file. The method is convenient to use, low in cost and can effectively and accurately detect the copy number of the spinal muscular atrophy virulence gene, the small segment insertion and deletion, the point mutation, the noncoding region mutation and even the gene translocation, and brings help and breakthrough for the clinical diagnosis and scientific research on the spinal muscular atrophy.
Owner:代苒
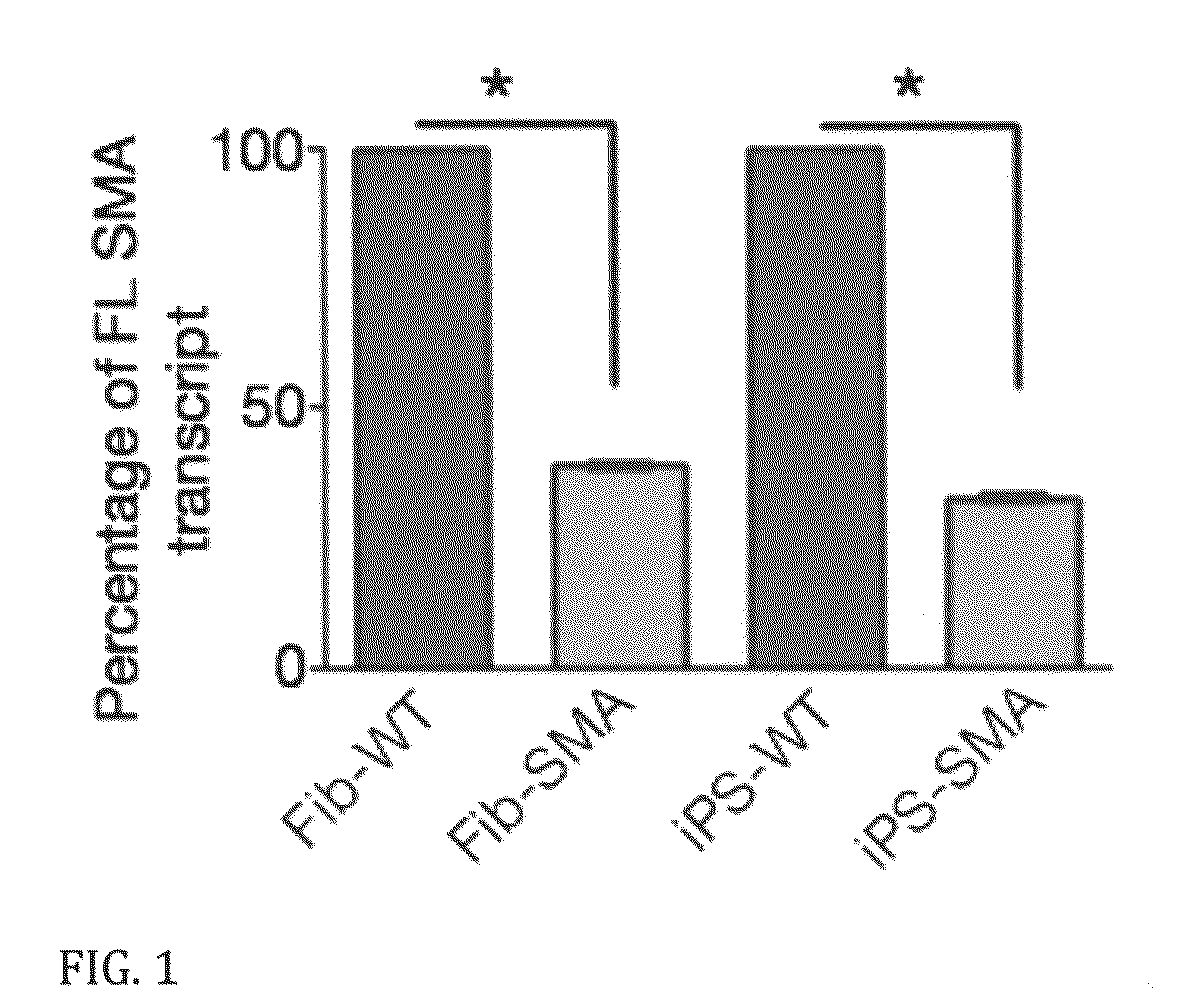
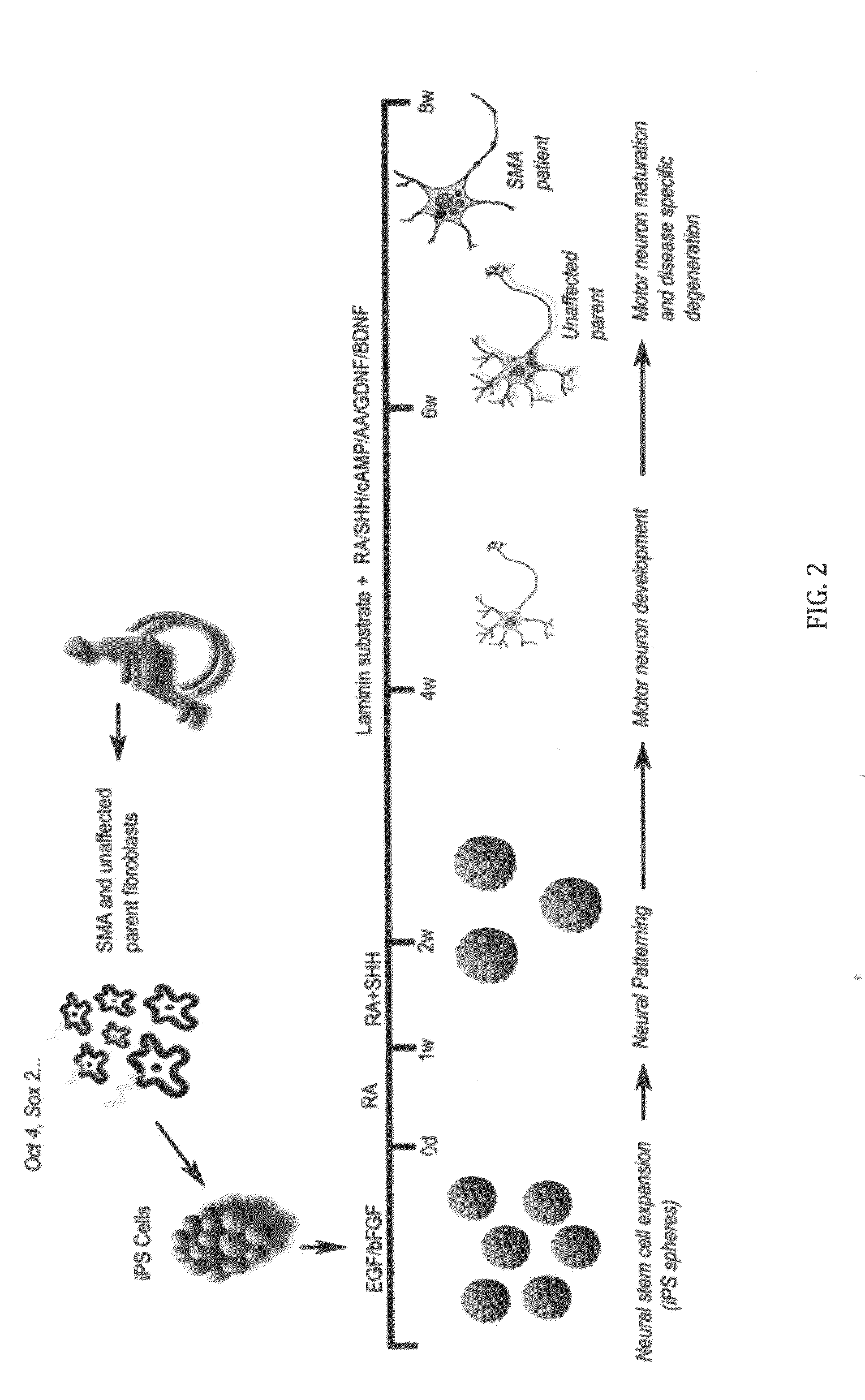
In vitro model of spinal muscular atrophy
A population of iPS cells derived from somatic cells from a spinal muscular atrophy patient is disclosed. In one embodiment of the invention, the cells have been cultured to produce neural cells. In another embodiment, the invention is a method of testing compounds for their ability to modify cellular SMN levels comprising the steps of obtaining a population of iPS cells derived from a spinal muscular atrophy patient or cells derived from the iPS cells, and examining the effect of a test compound on SMN levels.
Owner:WISCONSIN ALUMNI RES FOUND
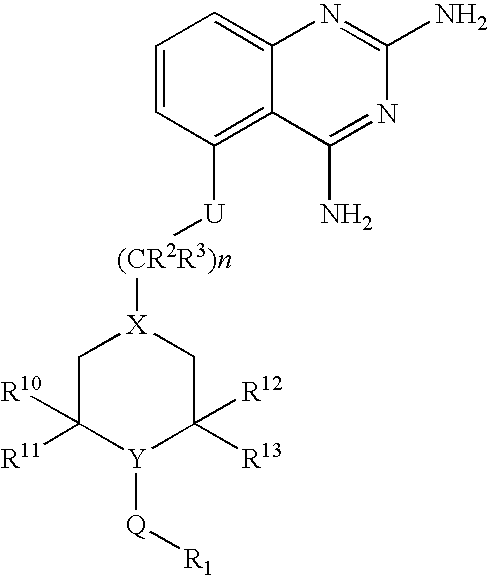
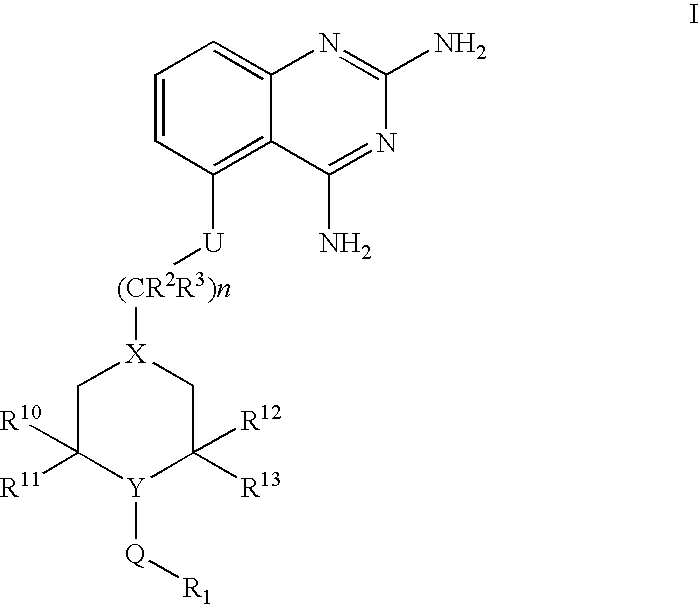
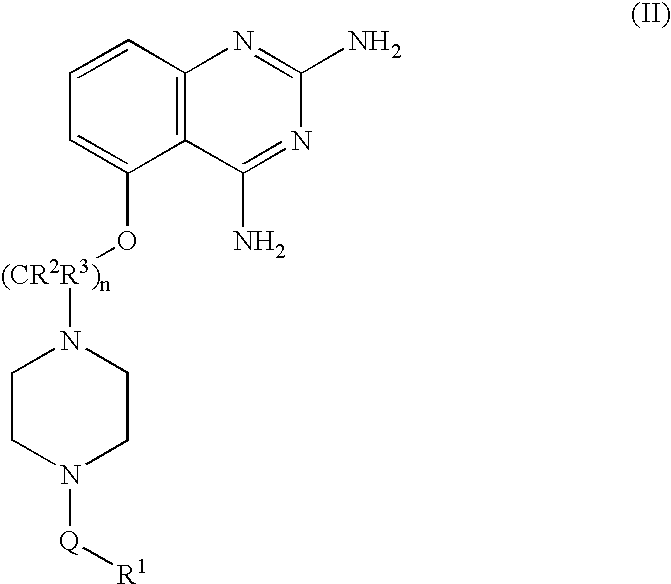
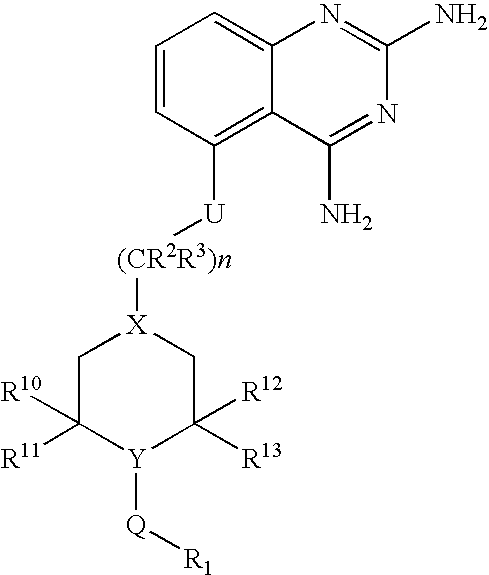
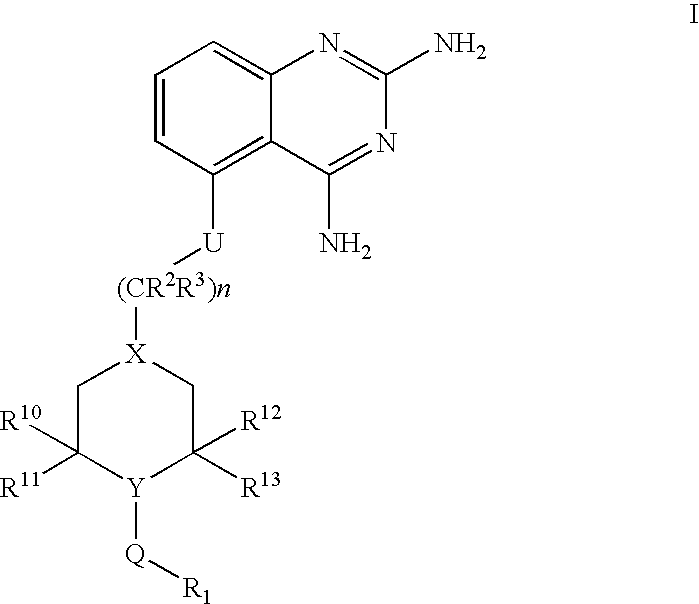
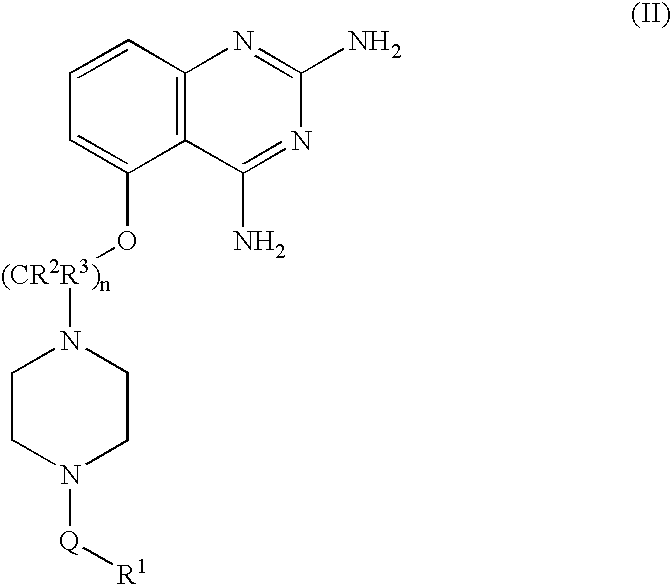
2,4-diaminoquinazolines for spinal muscular atrophy
InactiveUS20090042900A1Useful in treatmentOrganic active ingredientsBiocideDiaminoquinazolineAnesthesia
2,4-Diaminoquinazolines of formula (I) are provided herein and are useful for treating spinal muscular atrophy (SMA).
Owner:FAMILIES OF SPINAL MUSCULAR ATROPHY
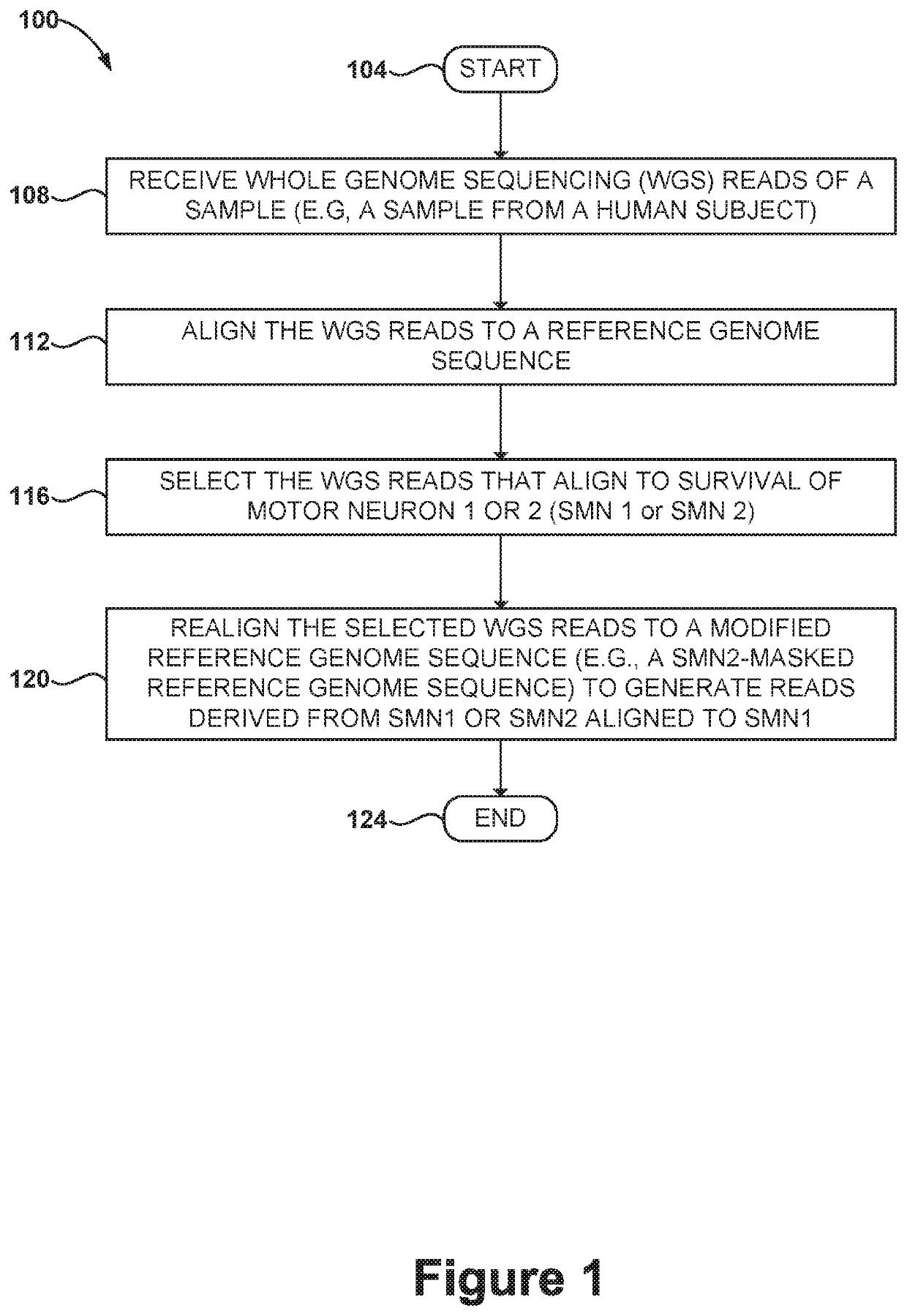
Methods and systems for determining paralogs
PendingUS20200087723A1Microbiological testing/measurementProteomicsReference genome sequenceMedicine
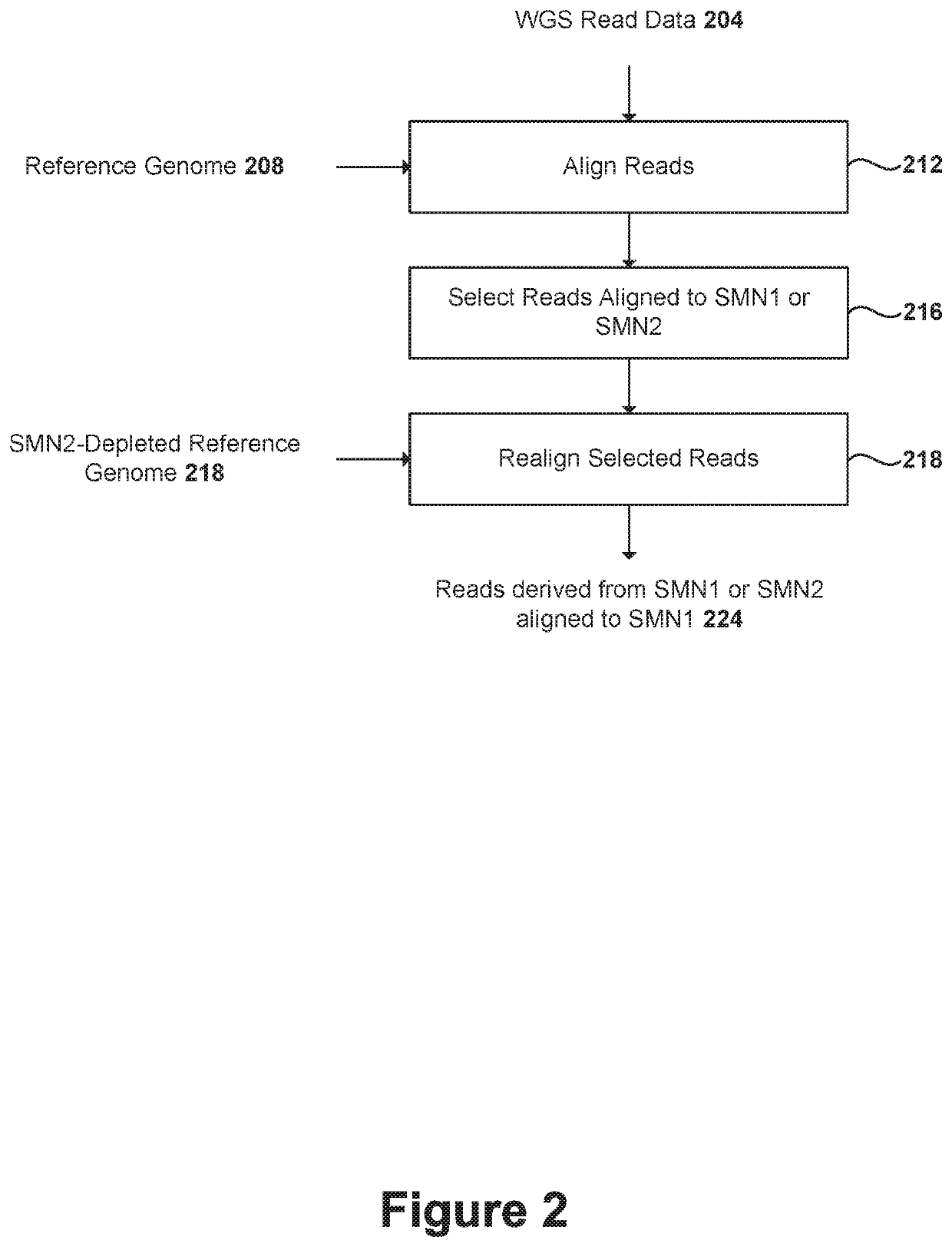
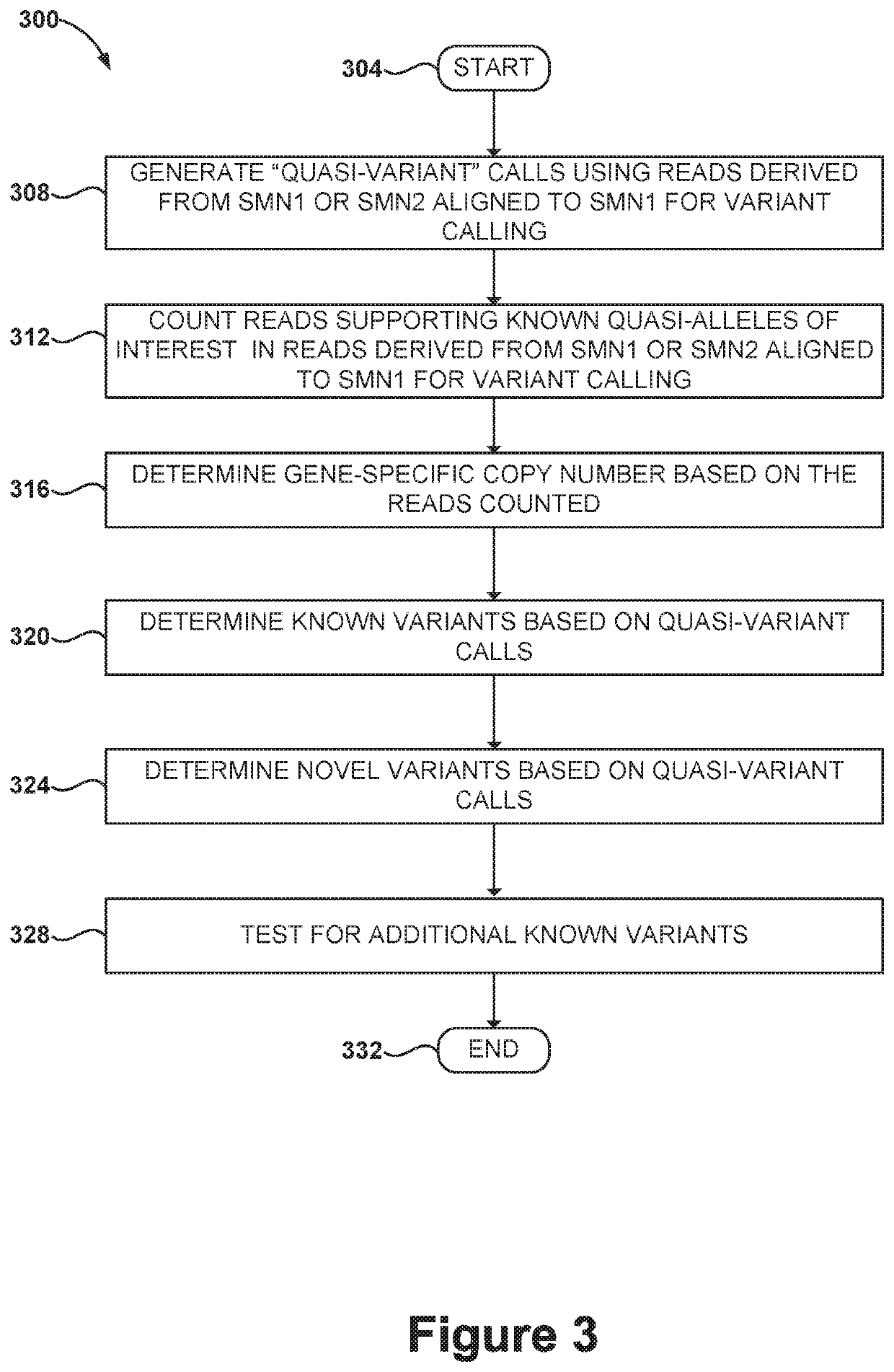
Disclosed herein are systems and methods for spinal muscular atrophy (SMA) diagnosis from whole genome sequencing data. In one embodiment, a method comprises aligning whole genome sequencing (WGS) reads of a subject's sample to a modified reference sequence such as a modified reference genome sequence. After counting the reads supporting quasi-alleles at select positions of the reference sequence, the method can adjust for coverage and determine a number of functional SMN1 gene copies. The method can determine affected or carrier status of the subject based on the copy number of functional SMN1 gene copies.
Owner:ILLUMINA CAMBRIDGE LTD +1
Optimization and extraction process of whole length polyclonal rabbit anti-human SMN antibody
InactiveCN1810835AStrong specificityImmunoglobulins against animals/humansRecombinant DNA-technologyProtein targetFhit gene
The present invention discloses the optimization and extraction process of whole length polyclonal rabbit anti-human SMN antibody, and belongs to the fields of early genic diagnosis of spinal muscular atrophy, SMN protein function research and disease prognosis judgment. The whole length polyclonal rabbit anti-human SMN antibody is prepared with the whole length SMN protein of 1-294 amino acids, and points to all functional regions of SMN protein. The preparation process includes preparation of whole length code region cDNA segment of health human, primer design and His gene fusion vector selection, preparation of the target gene segment, purification of His gene fusion protein and collection of target protein, and purification. The antibody of the present invention has certain specificity and sensitivity, and may play positive part in said fields.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
Assessing and treating spinal muscular atrophy
InactiveUS20200299772A1Early treatmentOrganic active ingredientsNervous disorderSpinal columnDisease
This document relates to methods and materials for assessing and / or treating a mammal (e.g., a human) having, or at risk of developing, a spinal condition (e.g., spinal muscular atrophy (SMA)). In some cases, a mammal can be identified as having, or as being likely to develop, a spinal condition (e.g., SMA), and, optionally, can be treated. For example, a mammal can be identified as having, or as being likely to develop, a spinal condition (e.g., SMA), based, at least in part, on the modification of nucleic acid that can encode a survival motor neuron (SMN) polypeptide (e.g., homozygous deletion of exon 7 of SMN1 nucleic acid encoding a SMN polypeptide and the genomic copy number of SMN2 nucleic acid encoding a SMN polypeptide) in a sample from a mammal and, optionally, can be treated.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
4-aminopyridine as a therapeutic agent for spinal muscular atrophy
It has been discovered that pharmacological inhibition of K+ channels (using the FDA-approved broad-spectrum K+ channel antagonist 4-AP) positively benefitted smn mutant phenotypes, a result that is consistent with the defective excitability of motor circuits by their interneuron or sensory neuron inputs being a critical consequence of SMN depletion. Based on these observations, certain embodiments of the invention are directed to methods of treatment of SMA by administering therapeutically effective amounts of one or more potassium channel antagonists, including 4-aminopyridine, 4-(dimethylamino)pyridine, 4-(methylamino)pyridine, and 4-(aminomethyl)pyridine. Other embodiments are directed to new pharmaceutical formulations comprising two or more potassium channel antagonists.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
CRISPR-Cas system for diagnosing spinal muscular atrophy and application thereof
ActiveCN111808947ADesign freedomSave operating timeMicrobiological testing/measurementAgainst vector-borne diseasesSpinal cordBioinformatics
The invention relates to a CRISPR-Cas system, in particular to a CRISPR-Cas system for diagnosing spinal muscular atrophy and application thereof. The CRISPR-Cas system for diagnosing spinal muscularatrophy disclosed by the invention comprises Cas14a1, crRNA, ssDNA and FQ-probe. A method based on combination of CRISPR / Cas14a1 and asymmetric PCR constructed by the invention can perform gene detection for SMA patients, and can be used for specifically detecting the mutation condition of SMN1 gene. Different from an existing CRISPR / Cas12a SMA diagnosis technology, the kit can also distinguish SMN2 gene interference so as to judge whether a detected person suffers from the spinal muscular atrophy or not, and has the characteristics of simple and convenient operation, high specificity and lowcost.
Owner:SHANGHAI PINPOINT MEDICAL TECH CO LTD
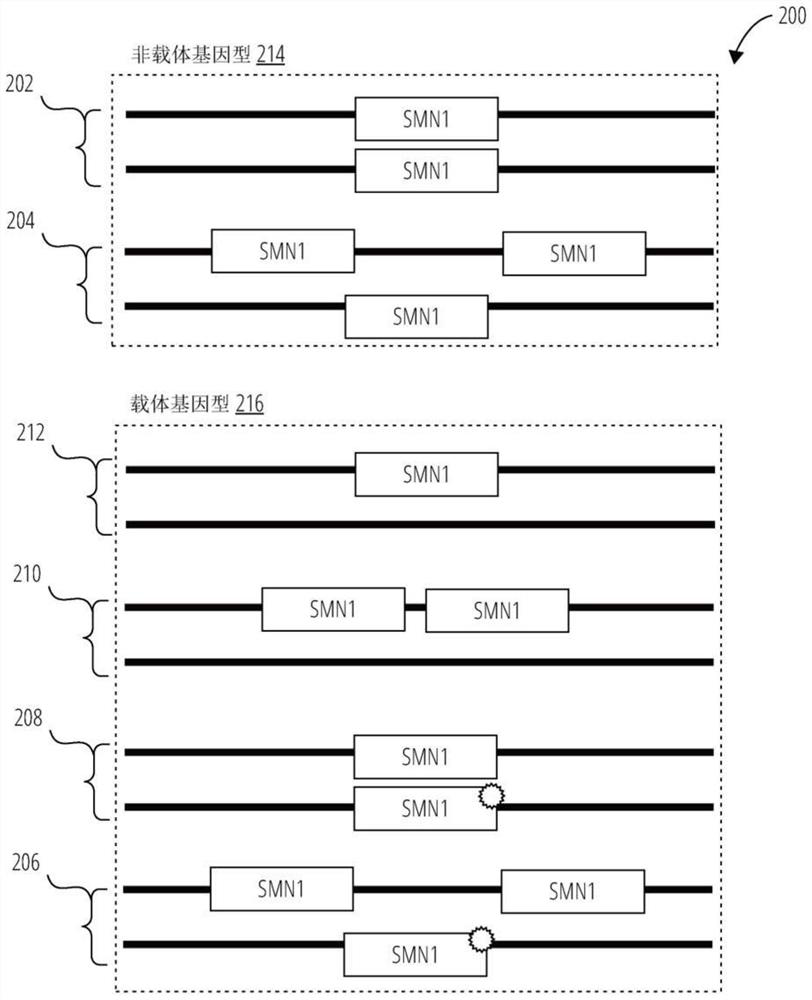
Array based method and kit for determining copy number and genotype in pseudogenes
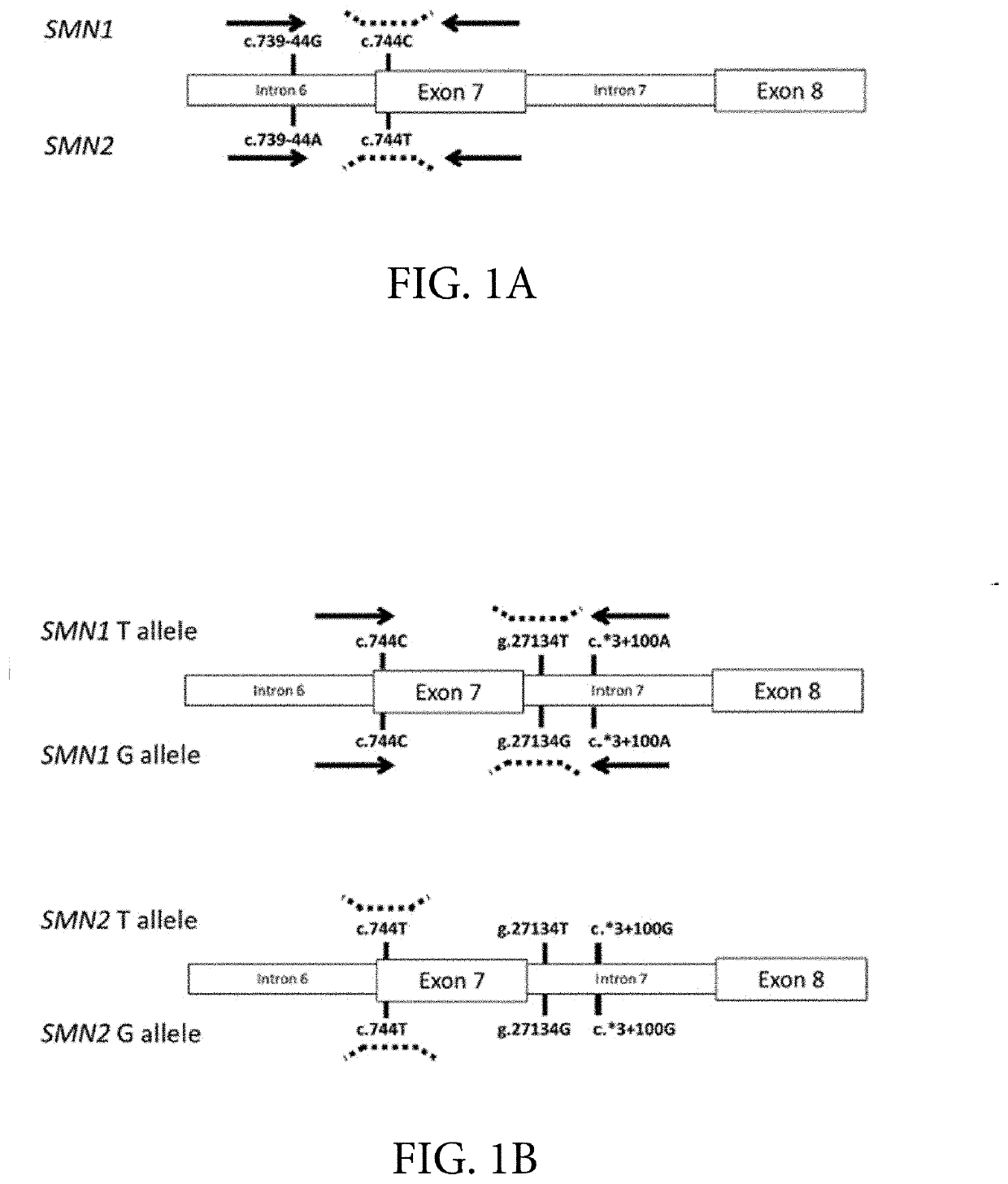
Provided herein are methods and associated compositions, kits, systems, devices and instruments useful for genetic analysis where there i s / a re a sequence(s) similar to the gene of interest in a sample, e.g. for determining the spinal muscular atrophy (SMA) carrier status. In the methods, a combined copy number for related genes (e.g., a gene of interest and its pseudogene, e.g. SMN1 and SMN2) can be determined via an assay. In addition, relative amounts of the related genes, i.e., a ratio of the related genes can be determined via the assay. Using the data of the combined copy number and the ratio of the related genes, the genotype of the gene of interest (as well as its pseudogene(s), if desired) can be determined with high accuracy.
Owner:AFFYMETRIX INC
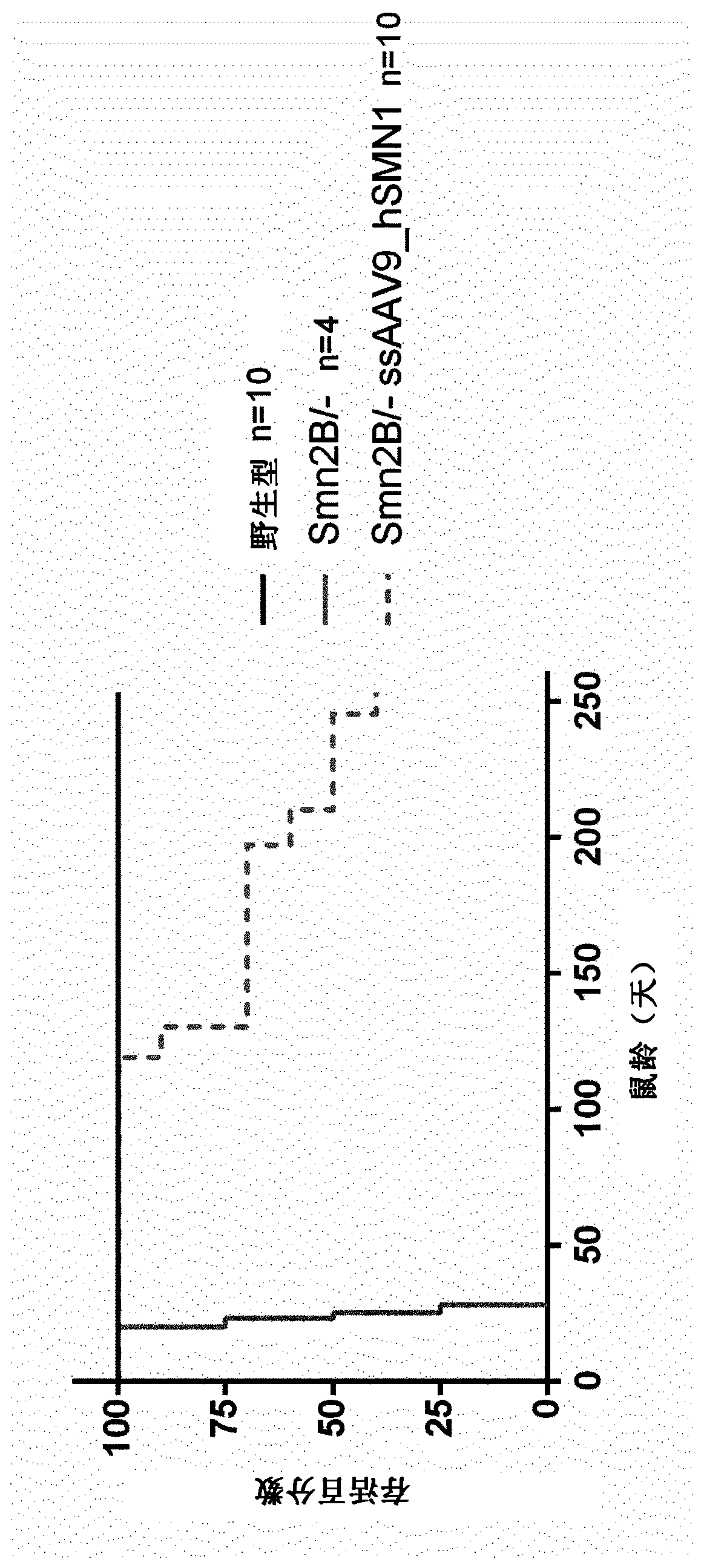
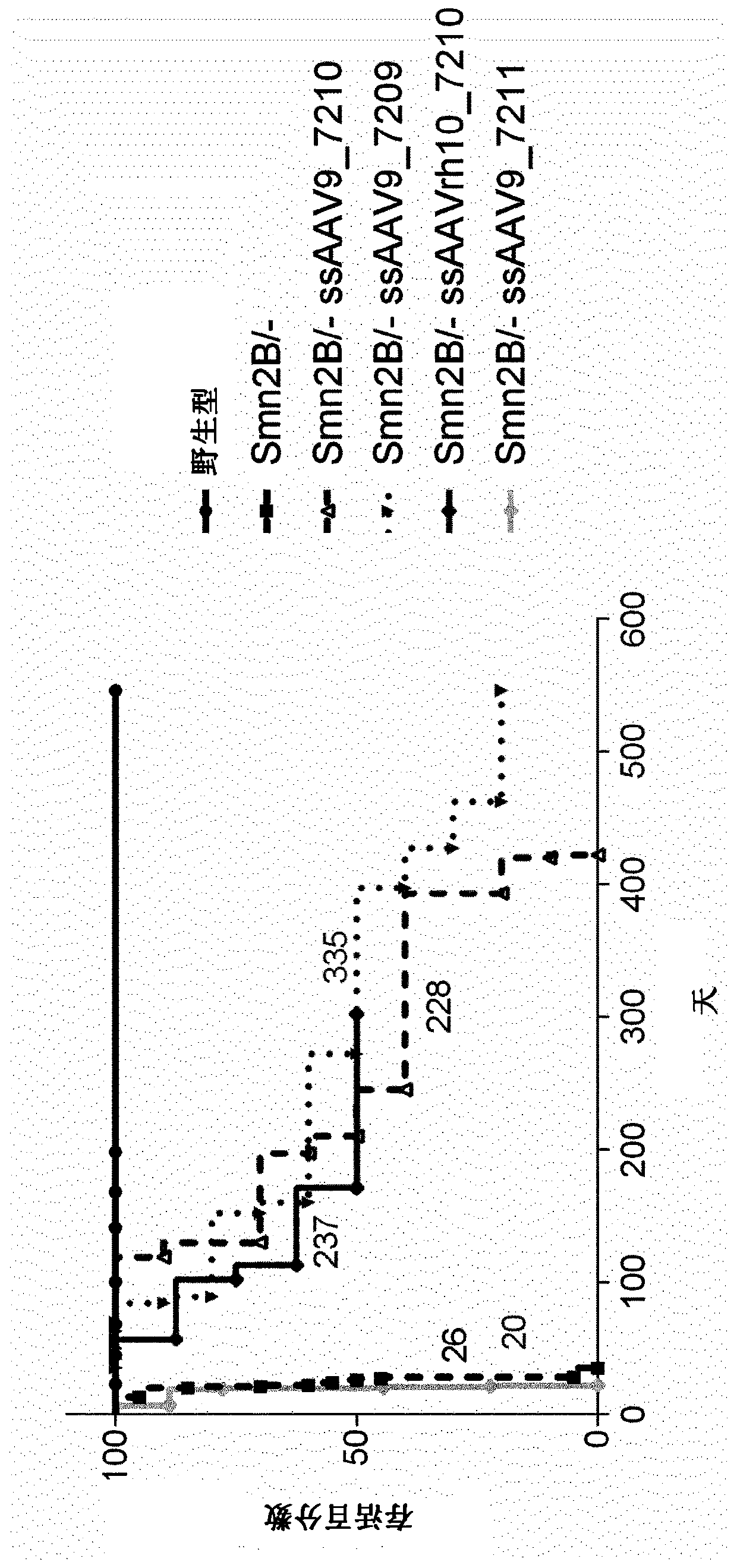
Treatment of spinal muscular atrophy
The present invention relates to a recombinant adeno-associated virus (rAAV) vector comprising a serotype 9 or rh10 AAV capsid, for use in a method for the treatment of spinal muscular atrophy (SMA).
Owner:GENETHON +2
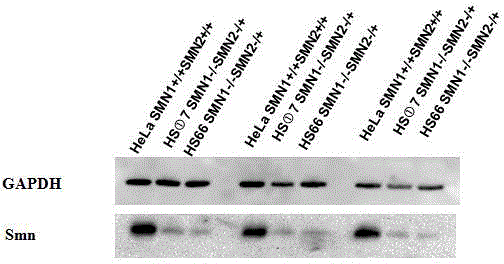
Method of knocking out spinal muscular atrophy SMN genes and cell model
ActiveCN103911346AHigh expressionSimple screening methodVector-based foreign material introductionForeign genetic material cellsAURKA GeneSpinal cord
The invention relates to a method of knocking out spinal muscular atrophy SMN genes and a cell model. The cell model is constructed by adopting the following steps: (1) constructing and designing zinc finger nuclease plasmids for knocking out the SMN genes; (2) transferring the constructed ZFN plasmids for knocking out the SMN genes into mammalian cells by using a lipidosome method; (3) detecting the effectiveness of the ZFN gene knockout by using a genome PCR product of a ZFN peculiar knockout site and DNA sequence determination; (4) specific to the cells, the ZFN genes of which are effectively knocked out, carrying out monoclonal separation and identifying cell knockout types one by one; (5) carrying out mRNA level identification on gene knockout cell strains; (6) carrying out protein expression level identification on the gene knockout cell strains. The spinal muscular atrophy pathology cell model can be used for research of SMA pathogenesis; medicines can be screened by detecting the level of protein expression of SMN compounds in the cell model.
Owner:江苏雄鸣医药科技有限公司
Systems and methods for providing improved prediction of carrier status for spinal muscular atrophy
Systems and methods of improved genetic mutation carrier screening may include, for a plurality of genetically similar genes in a reference genome, the plurality of genetically similar genes comprising a functional gene and a non-functional gene, masking the non-functional gene from the reference genome; aligning a plurality of functional gene reads and a plurality of non-functional gene reads of a patient's genetic sequence to the functional gene in the reference genome; tallying, at a first polymorphic locus-of-interest on each aligned read, a respective nucleotide type, wherein functional gene reads comprise a different nucleotide type than non-functional gene reads at the first polymorphic locus-of-interest; and calculating, based at least in part on a result of the tallying, a first gene ratio, wherein the first gene ratio indicates a first ratio of functional gene reads to non-functional gene reads.
Owner:ANCESTRY COM DNA
2,4-diaminoquinazolines for spinal muscular atrophy
2,4-Diaminoquinazolines of formula (I)are provided herein and are useful for treating spinal muscular atrophy (SMA).
Owner:FAMILIES OF SPINAL MUSCULAR ATROPHY
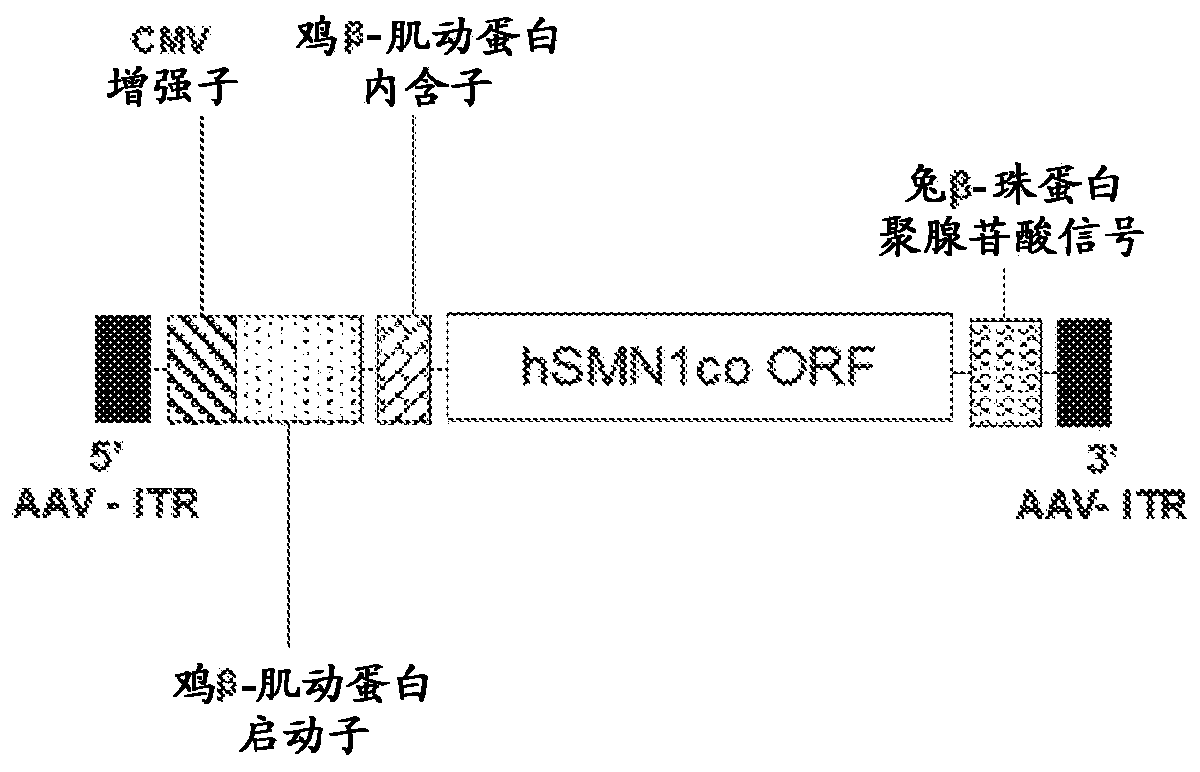
Compositions useful in treatment of spinal muscular atrophy
A rAAV vector is described herein which has an AAVhu68 capsid and at least one expression cassette in the capsid. The at least one expression cassette comprises nucleic acid sequences encoding a functional SMN protein and expression control sequences that direct expression of the SMN sequences in a host cell. Also provided are compositions containing this rAAVhu68.SMN vector and methods of using same for spinal muscular atrophy in a patient.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Modified human u1snrna molecule, a gene encoding for the modified human u1snrna molecule, an expression vector including the gene, and the use thereof in gene therapy of familial dysautonomia and spinal muscular atrophy
ActiveUS20170143847A1Low degree of conservationLow degreeSplicing alterationVectorsSpinal muscular atrophiesNucleotide
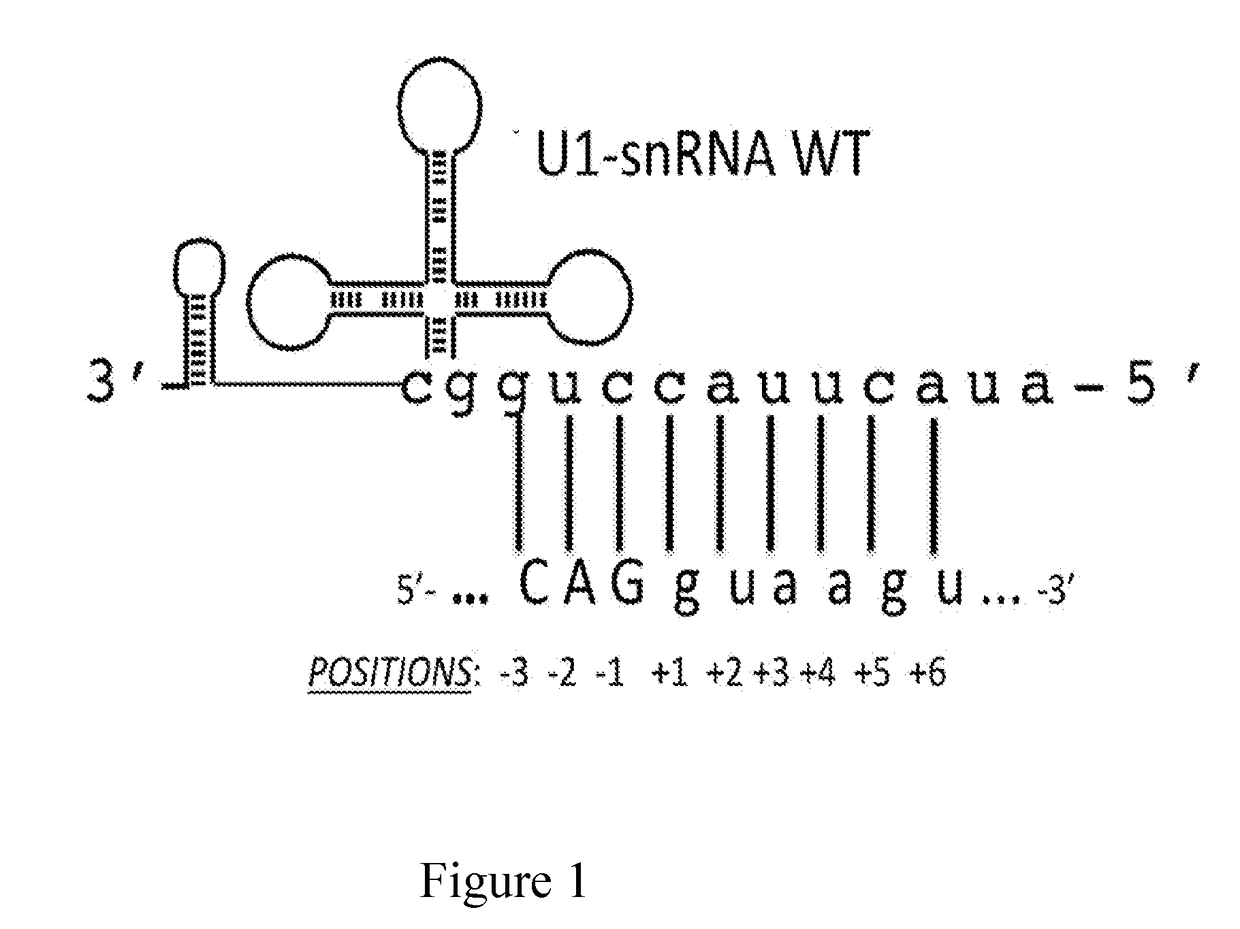
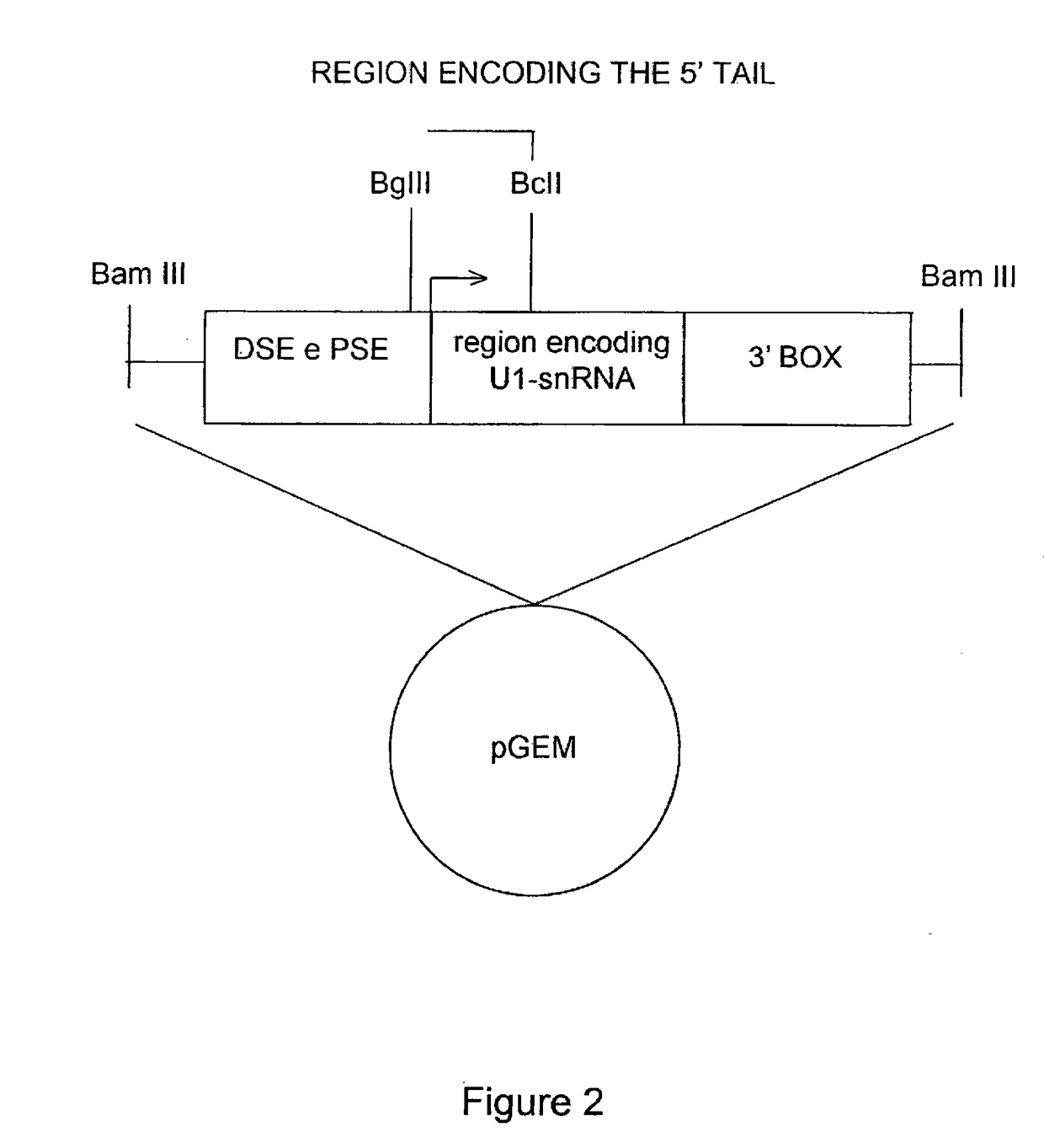
The invention provides a modified human U1snRNA molecule, capable of correcting the skipping of an exon caused by a mutation localized in the sequence comprised between 50 base pairs upstream and 20 base pairs downstream of an exon, wherein a portion of a single-stranded nucleotide sequence of the 5′ region of the wild-type human U1snRNA is replaced by a single-stranded binding nucleotide sequence, wherein the binding nucleotide sequence is selected from the group consisting of: uggcgcuua, aauggcgcu, aguacaauggcgc (SEQ ID NO: 87), gcaaacaguacaau (SEQ ID NO: 88), ucgcaaacaguaca (SEQ ID NO: 89), gcaaacagu, cuagucgcaaac (SEQ ID NO: 90), uacaaaaguaagauuca (SEQ ID NO: 83), aaaccauaaaguuuuacaa (SEQ ID NO: 84) and caaaccauaaaguuuua (SEQ ID NO: 96).
Owner:UNIV DI FERRARA
Primer group and kit for single-tube detection of human spinal muscular atrophy
PendingCN110885878AMultiple analysis informationReduce Amplification BiasMicrobiological testing/measurementDNA/RNA fragmentationPhysiologyNucleotide
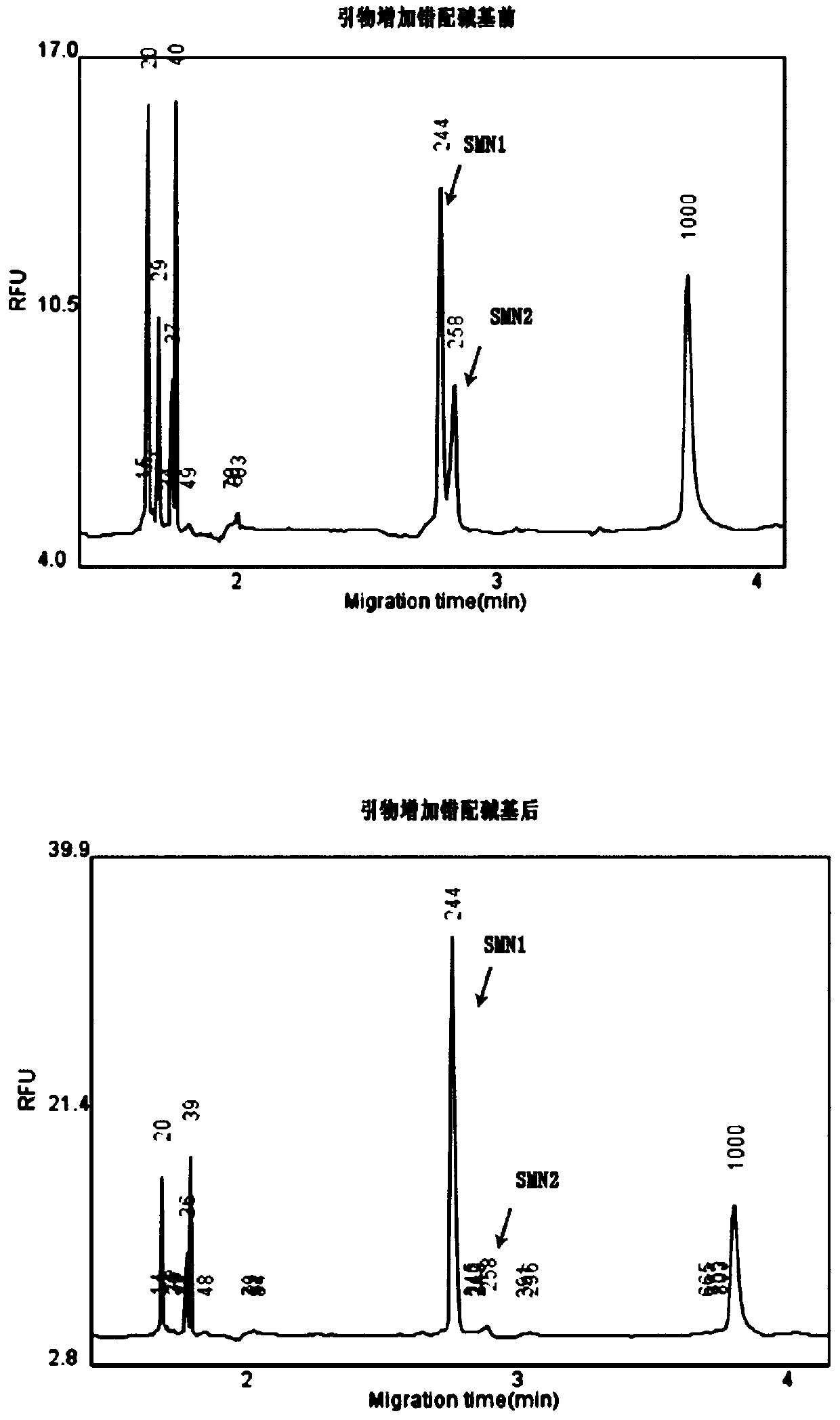
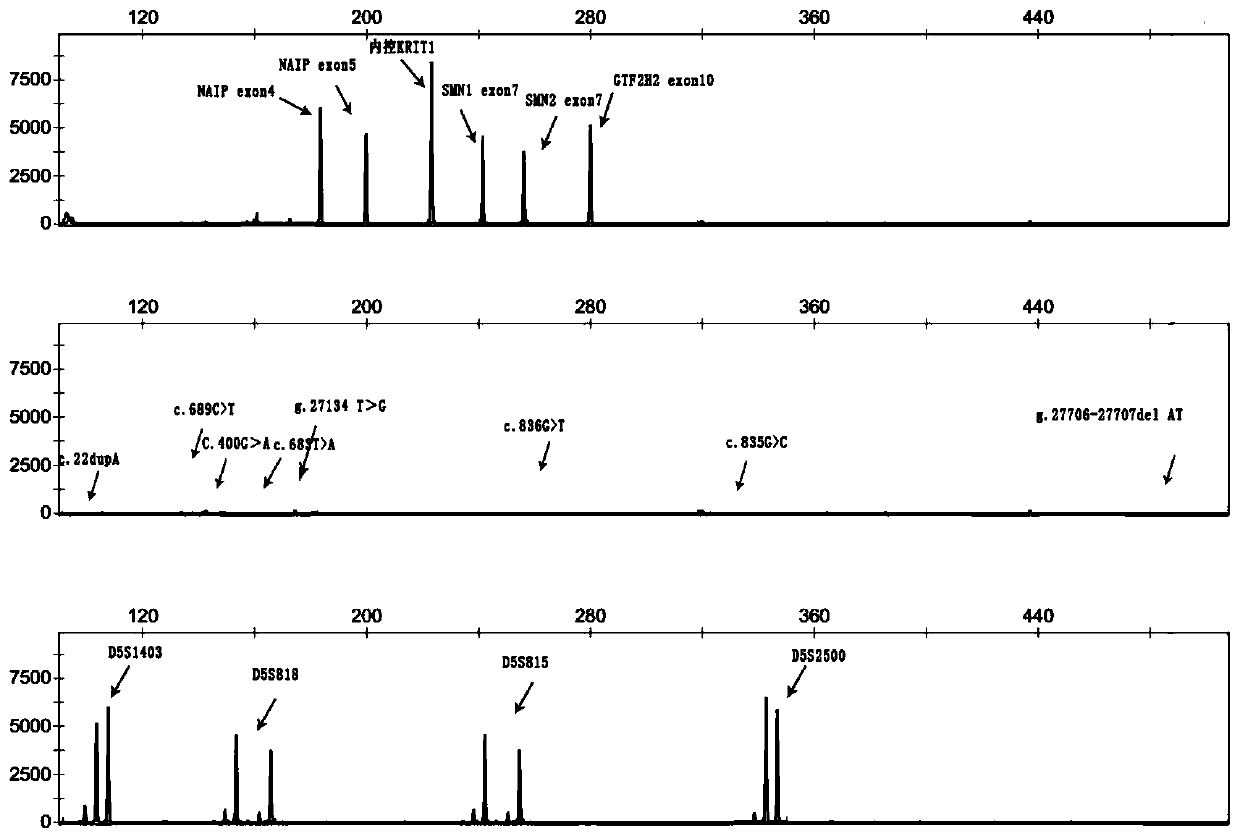
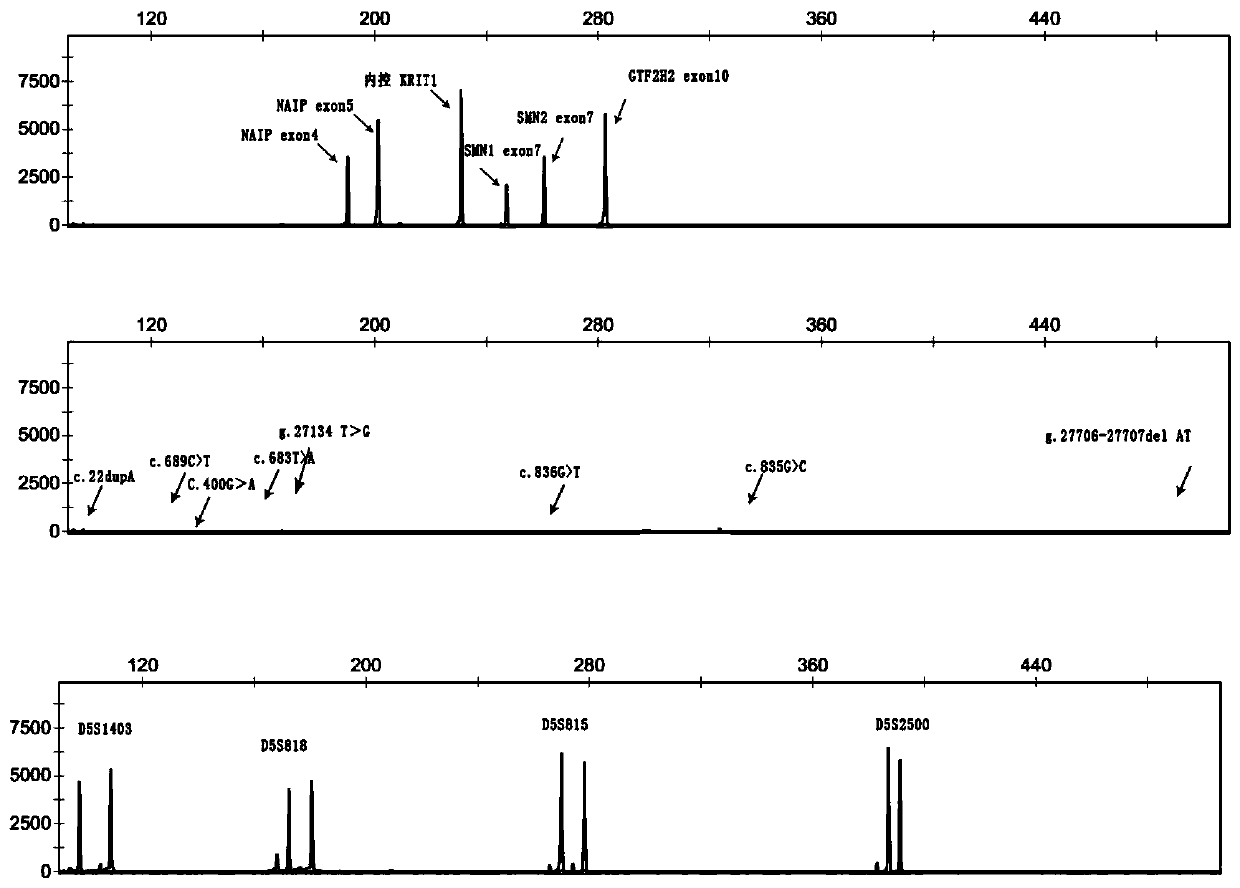
The invention discloses a primer group and a kit for detecting copy number and point mutation of a single-tube human spinal muscular atrophy related gene and typing a carrier. The nucleotide sequencesof the primer group are shown as SEQ ID NO. 1 to 38. The primer group is reasonable in design and high in specificity. The amplification bias is reduced, and the quantification accuracy is ensured. An identification sequence is introduced to realize identification of the conversion condition of the seventh exon of the SMN1 and the SMN2. UDG enzyme is introduced, and amplification products are cut, so as to avoid pollution. Clinical applications such as SMN1 / 2 gene copy number detection of human amyotrophy, SNP site detection of SMN1 / 2 Chinese population, NAIP and GTF2H2 gene copy number detection, typing of patients (I- VI types) and carriers ('1 + 0' and '2 + 0') and the like are simultaneously realized by the single tube. The method and the kit are rapid in detection, accurate in result, appropriate in cost, high in clinical occupancy of applicable instruments and suitable for clinical application.
Owner:GUANGZHOU DARUI BIOTECH
Digital PCR kit for detecting spinal muscular atrophy and application thereof
ActiveCN112522389AImprove accuracyImprove reliabilityMicrobiological testing/measurementReference genesCarrier screening
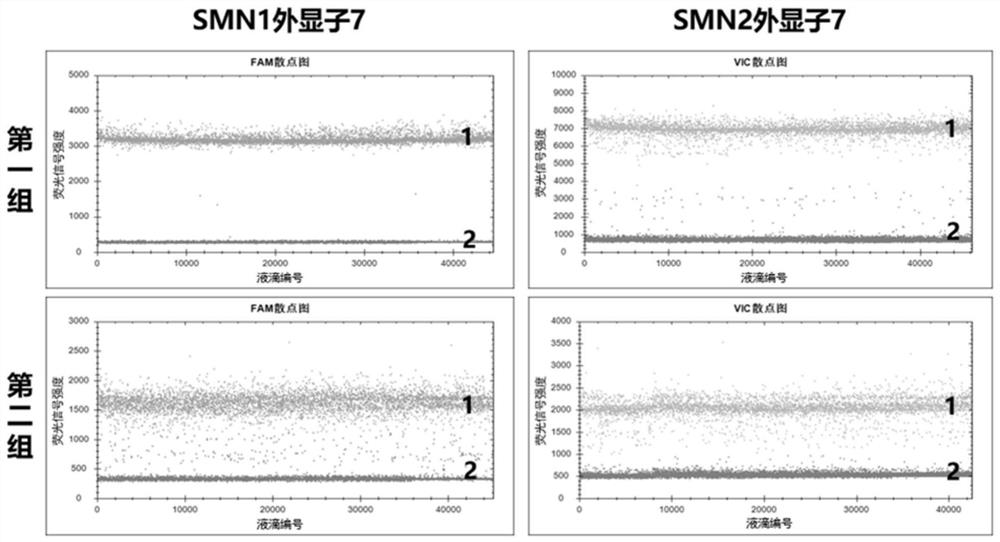
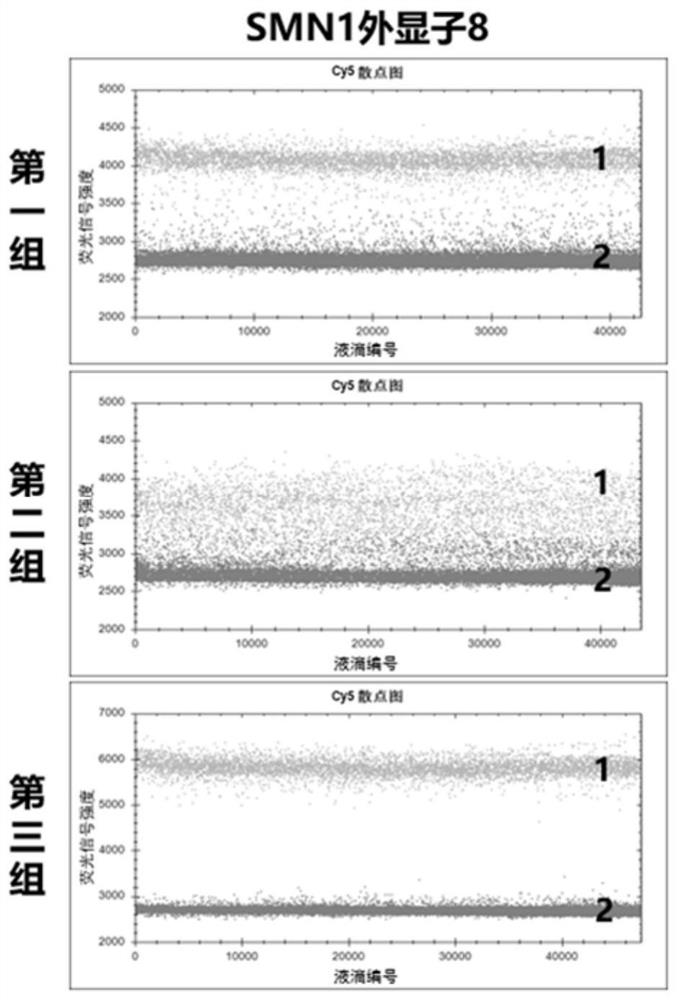
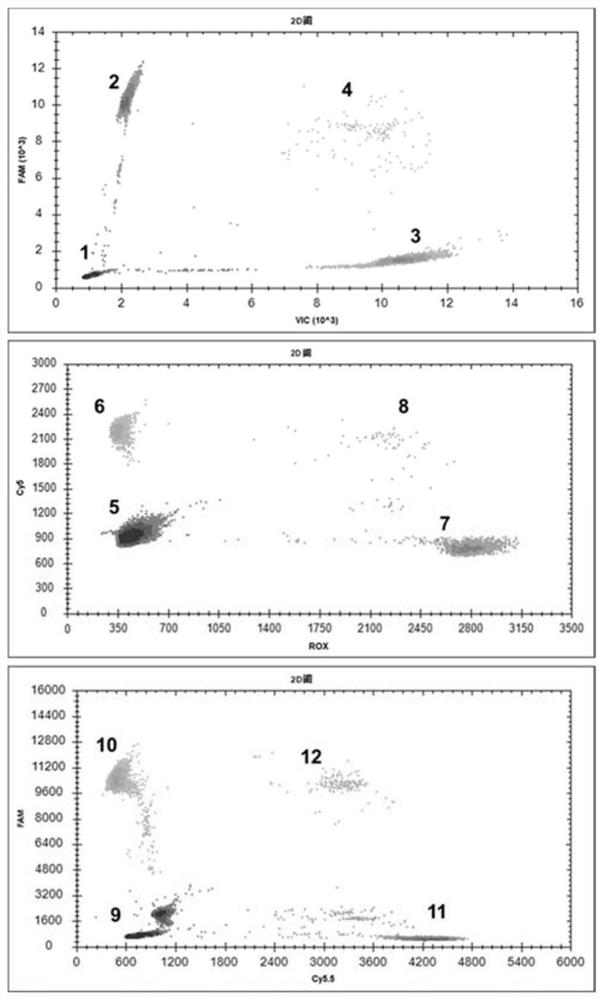
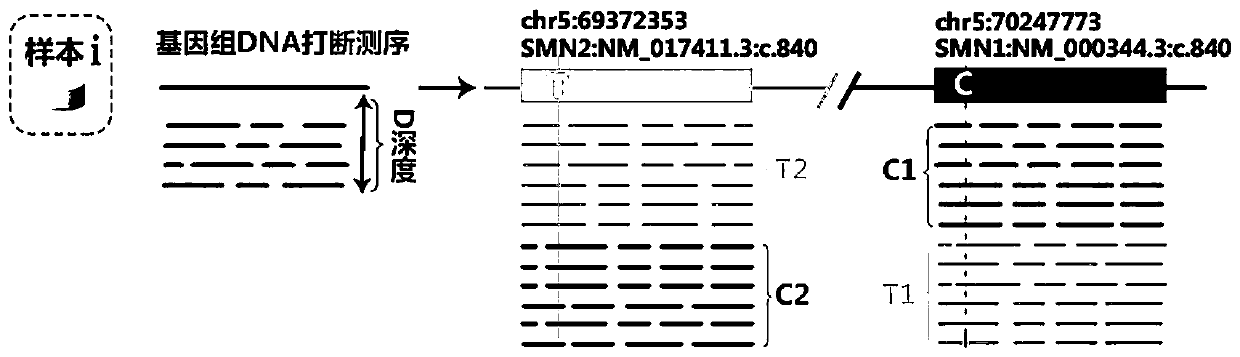
The invention provides a digital PCR kit for detecting spinal muscular atrophy. The digital PCR kit is a digital PCR kit for simultaneously detecting an SMN1 exon 7, an SMN1 exon 8, an SMN2 exon 7 andan internal reference gene in a sample to be detected in a one-tube digital PCR reaction, and the SMN1 exon 7, the SMN1 exon 8, the SMN2 exon 7 and the internal reference gene are simultaneously detected in at least four fluorescence channels. The kit disclosed by the invention is based on a digital PCR technology, can be used for quantitatively detecting the copy numbers of the SMN1 exons 7 and8 and the SMN2 in one tube, and can be applied to SMA clinical definite diagnosis, carrier screening and disease typing.
Owner:TSINGHUA UNIV +1
Method and system for detecting SMN1 gene mutation by means of high-throughput sequencing
ActiveCN111292804AAvoid mistakesMicrobiological testing/measurementMedical automated diagnosisGenes mutationDisease
The invention relates to a device, a method and a system for detecting SMN1 gene mutation by analyzing a high-throughput sequencing result, in particular the homozygous deletion of a seventh exon of an SMN1 gene. The invention also relates to the use of the device, method and system according to the invention to diagnose spinal muscular atrophy (SMA) or differentially diagnose SMA and other diseases that are susceptible to confusion with the SMA phenotype, as well as to a machine-readable medium and a terminal device on which the method according to the invention is stored.
Owner:北京智因东方诊断科技有限公司
Compositions Useful in Treatment of Spinal Muscular Atrophy
A rAAV vector is described herein which has an AAVhu68 capsid and at least one expression cassette in the capsid. The at least one expression cassette comprises nucleic acid sequences encoding a functional SMN protein and expression control sequences that direct expression of the SMN sequences in a host cell. Also provided are compositions containing this rAAVhu68.SMN vector and methods of using same for spinal muscular atrophy in a patient.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Aav viral vectors and uses thereof
Disclosed herein are compositions comprising AAV9 viral vectors and methods of using them to treat SMA patients, e.g., Type II and Type III Spinal Muscular Atrophy (SMA) patients.
Owner:NOVARTIS AG
Compositions and methods for treating spinal muscular atrophy
The present provides methods for treating spinal muscular atrophy using a self-complementary recombinant adeno-associated virus (rAAV) viral particle comprising a transgene expressing SMN. In one aspect, the viral particles are administered the spinal column or cisterna magna in a human subject; for example, a pediatric human subject. Viral particles comprising AAV9 capsids are contemplated.
Owner:GENZYME CORP
Primers, probes and kits for quantitative detection of spinal muscular atrophy genes
ActiveCN104480206BEasy to detectAvoid false negativesMicrobiological testing/measurementDNA/RNA fragmentationSpinal muscular atrophiesFluorescence
The invention relates to the field of gene detection, and particularly relates to a primer, a probe and a kit for fluorescent quantitative detection on genes of spinal muscular atrophy (SMA). The kit comprises the primer and the probe for fluorescent quantitative detection on genes of SMA, wherein the primer includes 9 pairs of primers; the probe includes 15 probes. According to the primer, the probe and the kit for fluorescent quantitative detection on genes of SMA, a fluorescent quantitative PCR technology is utilized, common mutation site detection which is not found in existing patent technologies is added, so that, 16 types of SMA patients caused by SMN gene deletion, transformation and site mutation can be specifically detected in one time, and classification of SMA patients can be realized.
Owner:亚能生物技术(深圳)有限公司
Primer-probe combination for screening diseases, application of primer-probe combination, kit and detection method
ActiveCN111206092AScreening achievedLow costMicrobiological testing/measurementAgainst vector-borne diseasesCombined immunodeficiency diseaseSpinal cord
The invention discloses a primer-probe combination for simultaneously screening spinal muscular atrophy and primary immunodeficiency diseases, application of the primer-probe combination, a detectionkit and a detection method. The detection kit comprises primers and probes for screening and detecting a spinal muscular atrophy gene and primers and probes for screening and detecting genes (TREC andKREC) of the primary immunodeficiency diseases (advanced-case combined immunodeficiency disease and agammaglobulinemia). According to the primer-probe combination, the application of the primer-probecombination, the detection kit and the detection method, a multiple digital PCR detection system with high sensitivity, high accuracy, low cost and short cycle can be established, quantified detection on a copy number of a pathogenic gene of a hereditary disease, i.e., the spinal muscular atrophy (SMA), a copy number of an advanced-case combined immunodeficiency disease related marker TREC and acopy number of an agammaglobulinemia related marker KREC can be achieved simultaneously in one reaction, and thus, the above-mentioned three kinds of diseases are screened in one time.
Owner:上海捷易生物科技有限公司
Spinal muscular atrophy test
ActiveCN113151419BHas clinical application valueComprehensive detection rangeMicrobiological testing/measurementDNA/RNA fragmentationGenomic DNASpinal cord
The invention discloses a spinal muscular atrophy detection method, wherein the spinal muscular atrophy detection method includes extraction of sample genomic DNA, design and synthesis of amplification primers and detection probes, fluorescence quantitative PCR reaction, relative gene copy number Calculation and analysis of base mutation levels. The spinal muscular atrophy detection method of the invention can realize the simultaneous detection of the relative copy number of SMN1, the relative copy number of SMN2 and the point mutation of SMN1, the detection is comprehensive, the steps are simple, and the clinical application and popularization are easy.
Owner:深圳会众生物技术有限公司
And antisense oligonucleotide is used for targeting region where methylation site nt-290 of SMN2 promoter region is located
ActiveCN112662671AImprove expression levelOrganic active ingredientsNervous disorderTreatment targetsPromoter
The invention provides an antisense oligonucleotide targeting a region where a methylation site nt-290 of an SMN2 promoter region is located. The antisense oligonucleotide can be combined to a key methylation region of an SMN2 gene promoter region to promote the overall transcription level of an SMN2 gene and the expression level of SMN protein in spinal muscular atrophy patient cells, and can be used as a new treatment target in an SMN targeting treatment strategy.
Owner:首都儿科研究所
Phytoecdysones and the derivatives thereof for use in the treatment of neuromuscular diseases
PendingUS20220160732A1Limit muscular atrophy and aplasiaImprove survivalOrganic active ingredientsMuscular disorderDiseaseAmytrophic lateral sclerosis
Disclosed are 20-hydroxyecdysone and the derivatives thereof, intended for use in the treatment of a neuromuscular disease such as spinal muscular atrophy or amyotrophic lateral sclerosis, or more particularly in the treatment of a specific disorder of the motor neurons causing alterations in the muscular function occurring in the context of these neuromuscular diseases.
Owner:BIOPHYTIS +1
A CRISPR-Cas system for diagnosing spinal muscular atrophy and its application
ActiveCN110551812BImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMedicineSpinal cord
The invention discloses a CRISPR-Cas system for diagnosing spinal muscular atrophy. The system comprises Cas 12. Preferably, the CRISPR-Cas system includes Cas12, crRNA, dsDNA, and reporter RNA strands. At that same time, the invention also disclose application of the CRISPR-Cas system in that preparation of kit for diagnosis of spinal muscular atrophy. The CRISPR-CAS system can quickly detect themutation of SMN1 gene, the pathogenic gene of spinal muscular atrophy, and has the advantages of high specificity and sensitivity, intuitive analysis of results, accurate rate as high as 100%, etc. The CRISPR-Cas system is very suitable for large-scale clinical sample detection.
Owner:JINAN UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com