Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Hazardous drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
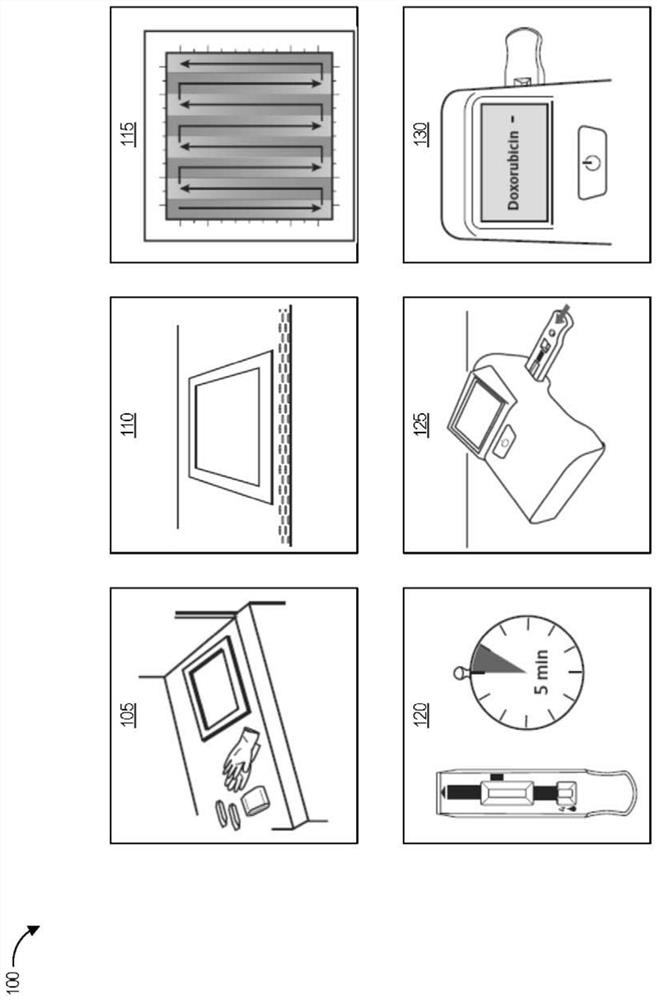
In pharmacology, hazardous drugs are drugs that are known to cause harm, which may or may not include genotoxicity (the ability to cause a change or mutation in genetic material). Genotoxicity might involve carcinogenicity, the ability to cause cancer in animal models, humans or both; teratogenicity, which is the ability to cause defects on fetal development or fetal malformation; and lastly hazardous drugs are known to have the potential to cause fertility impairment, which is a major concern for most clinicians. These drugs can be classified as antineoplastics, cytotoxic agents, biologic agents, antiviral agents and immunosuppressive agents. This is why safe handling of hazardous drugs is crucial.
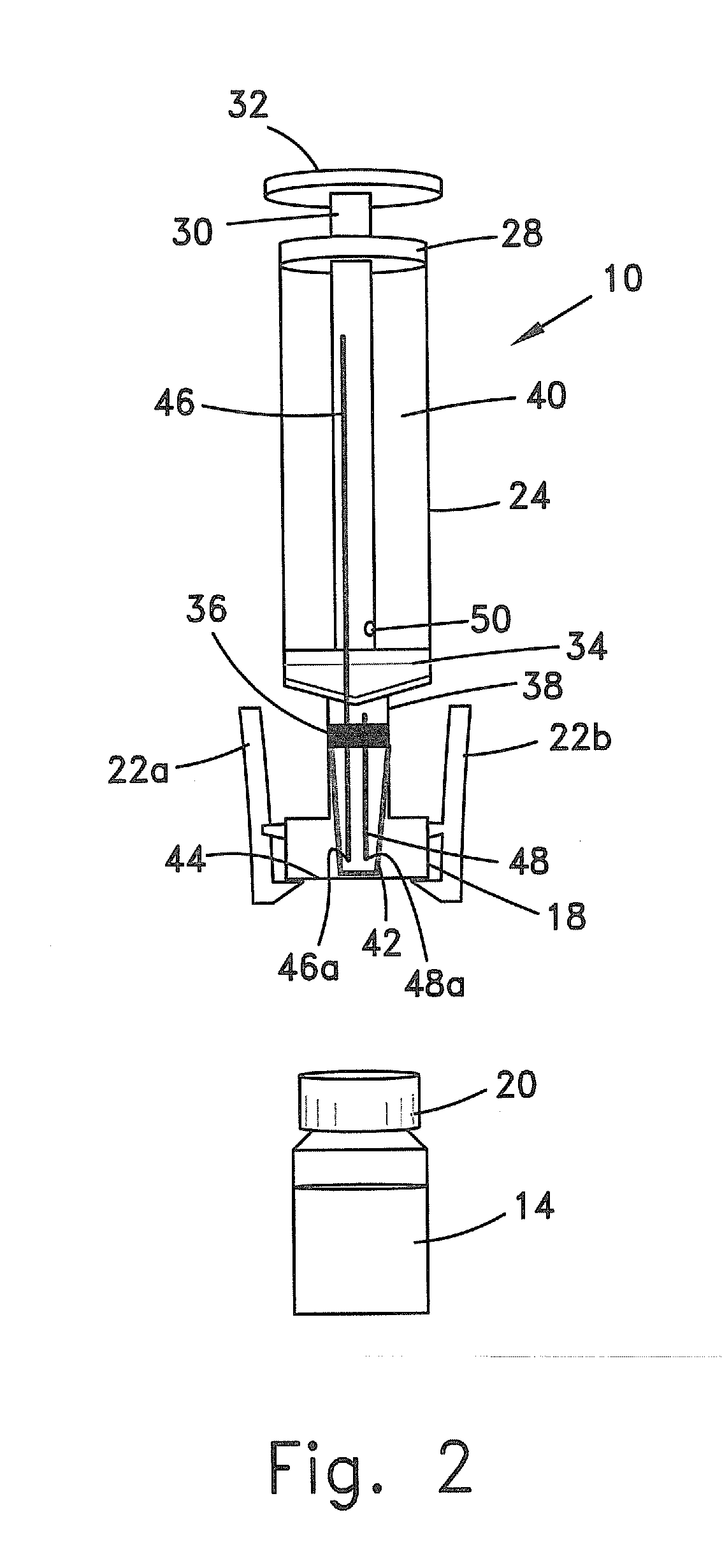
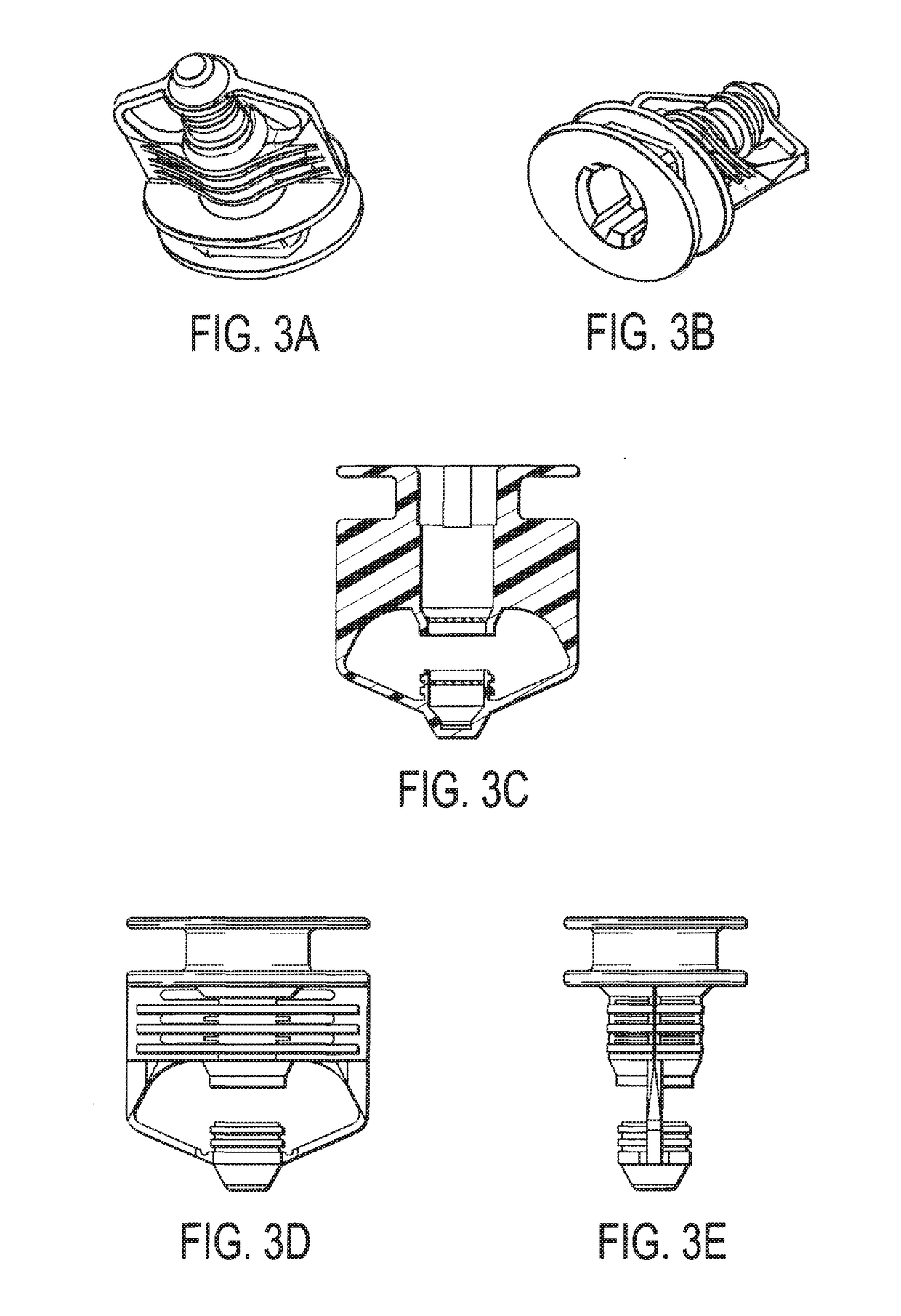
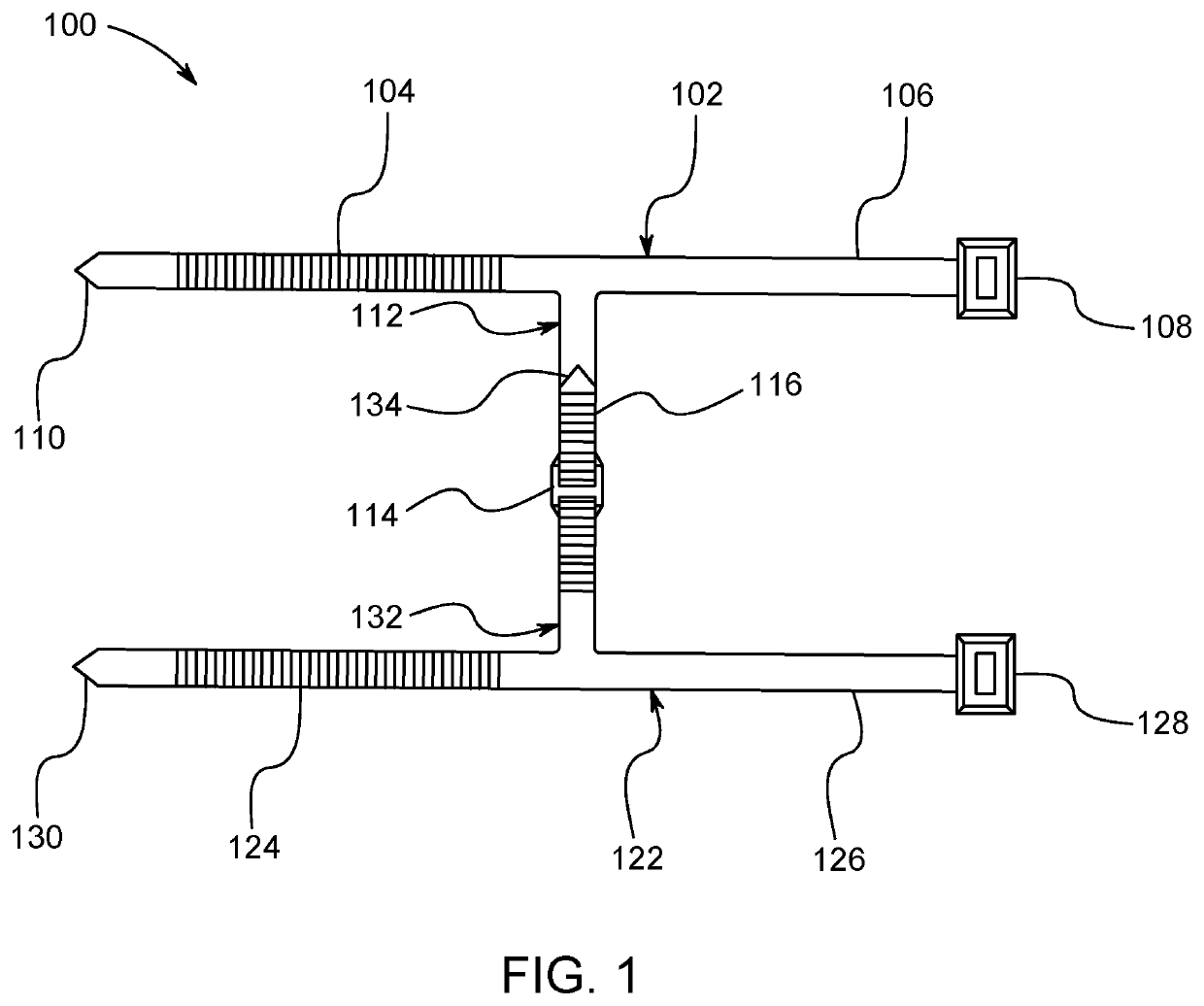
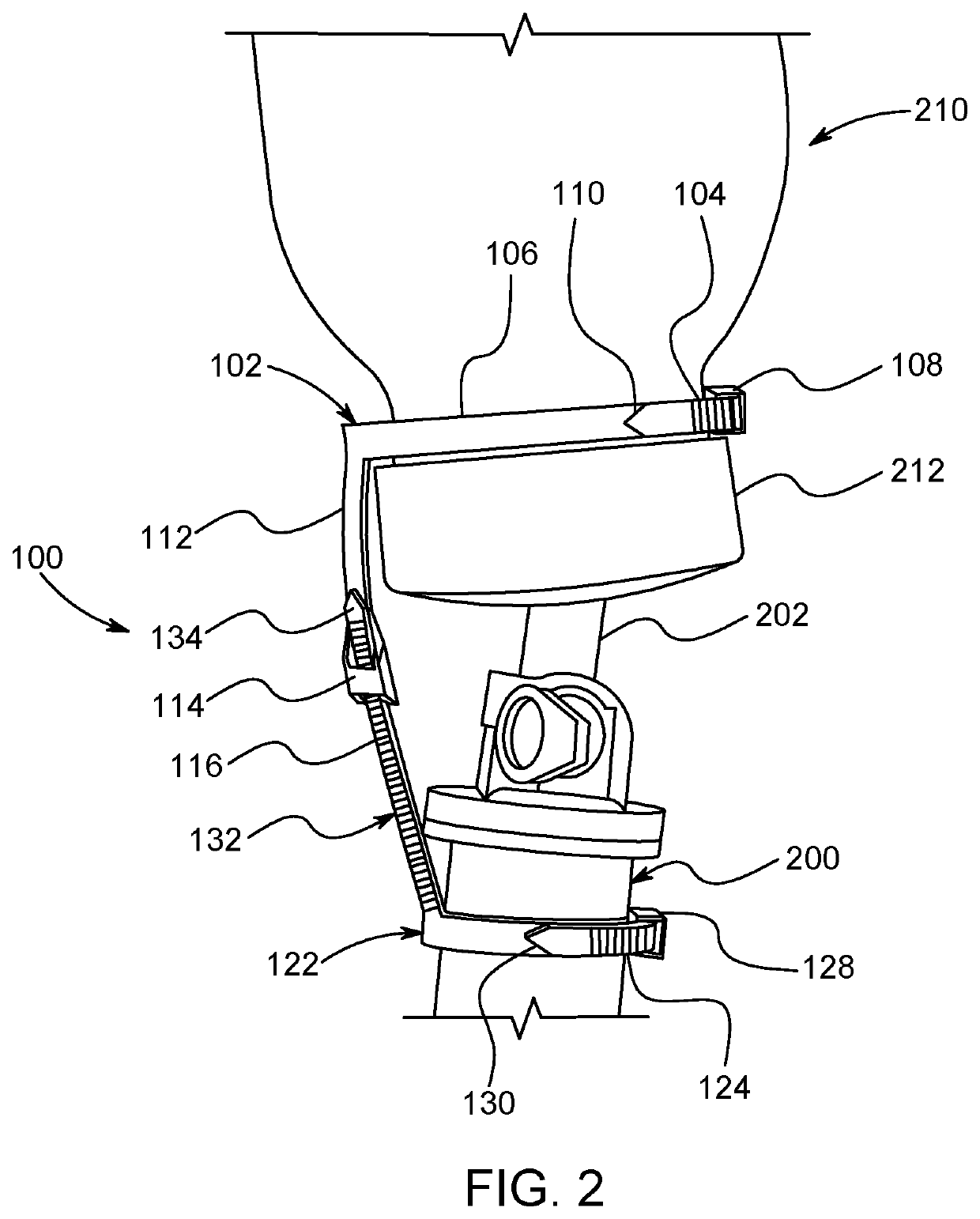
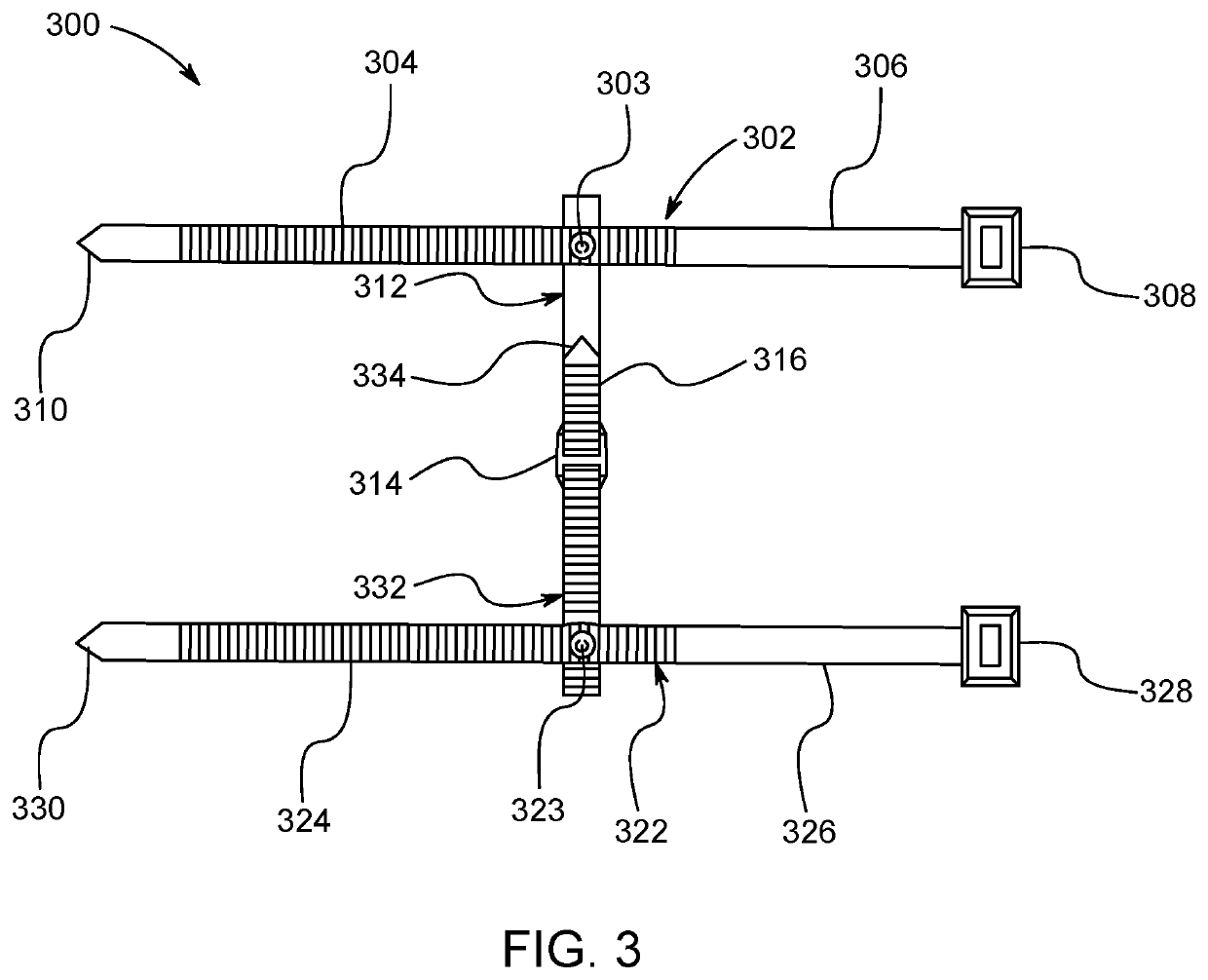
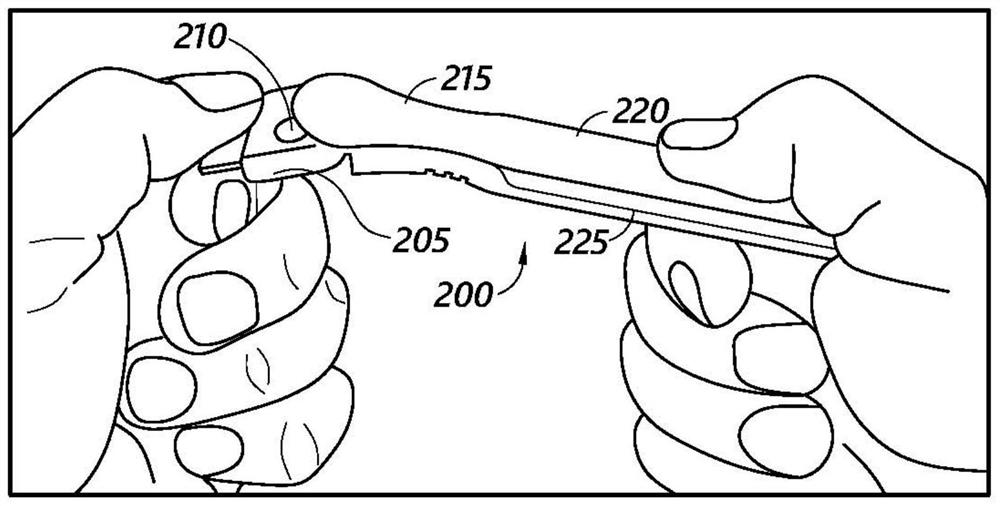
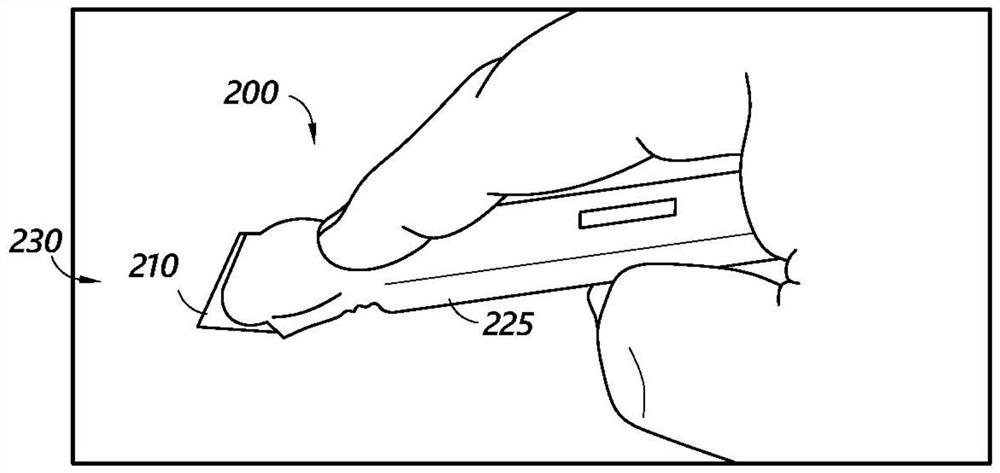
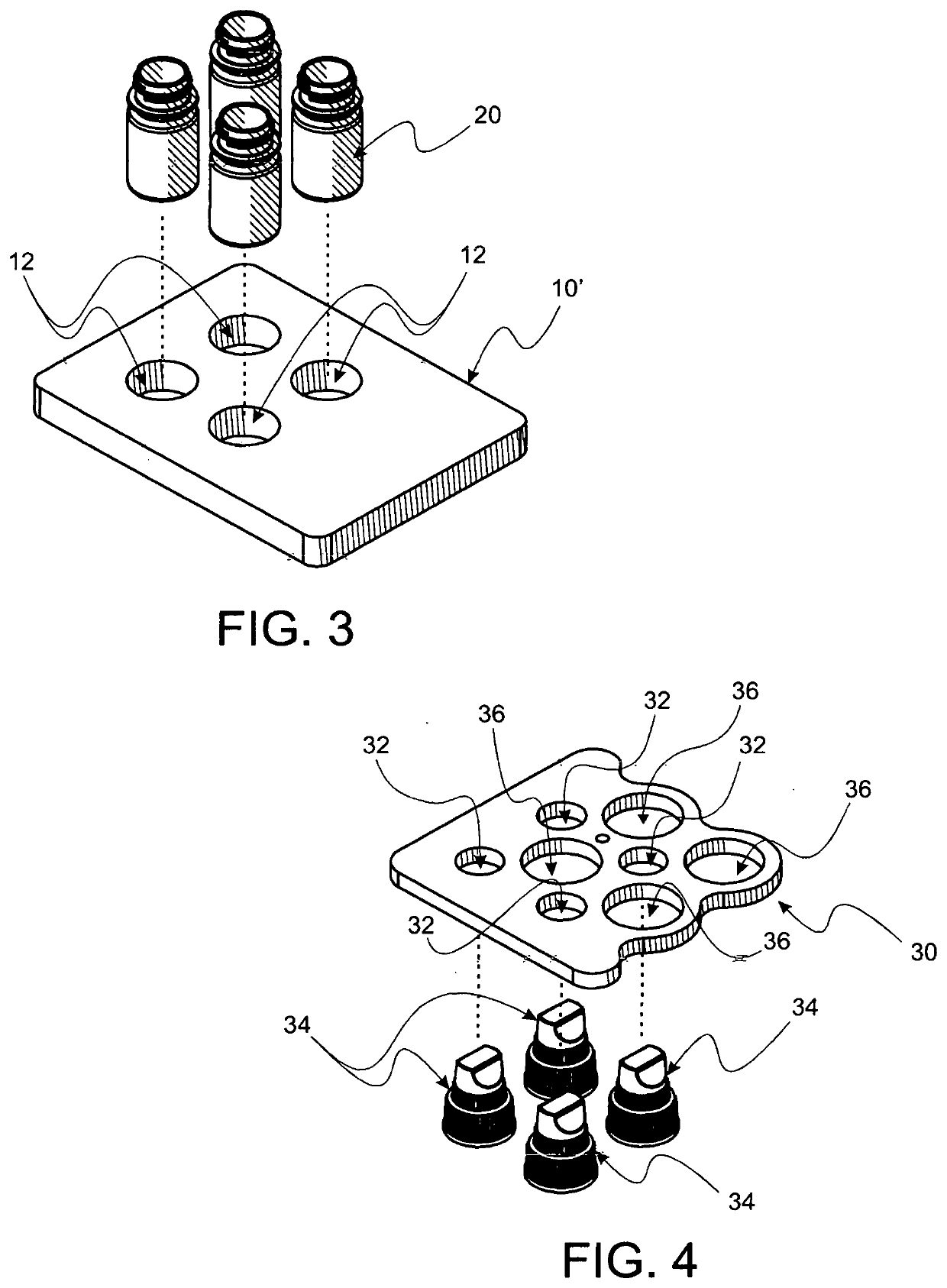
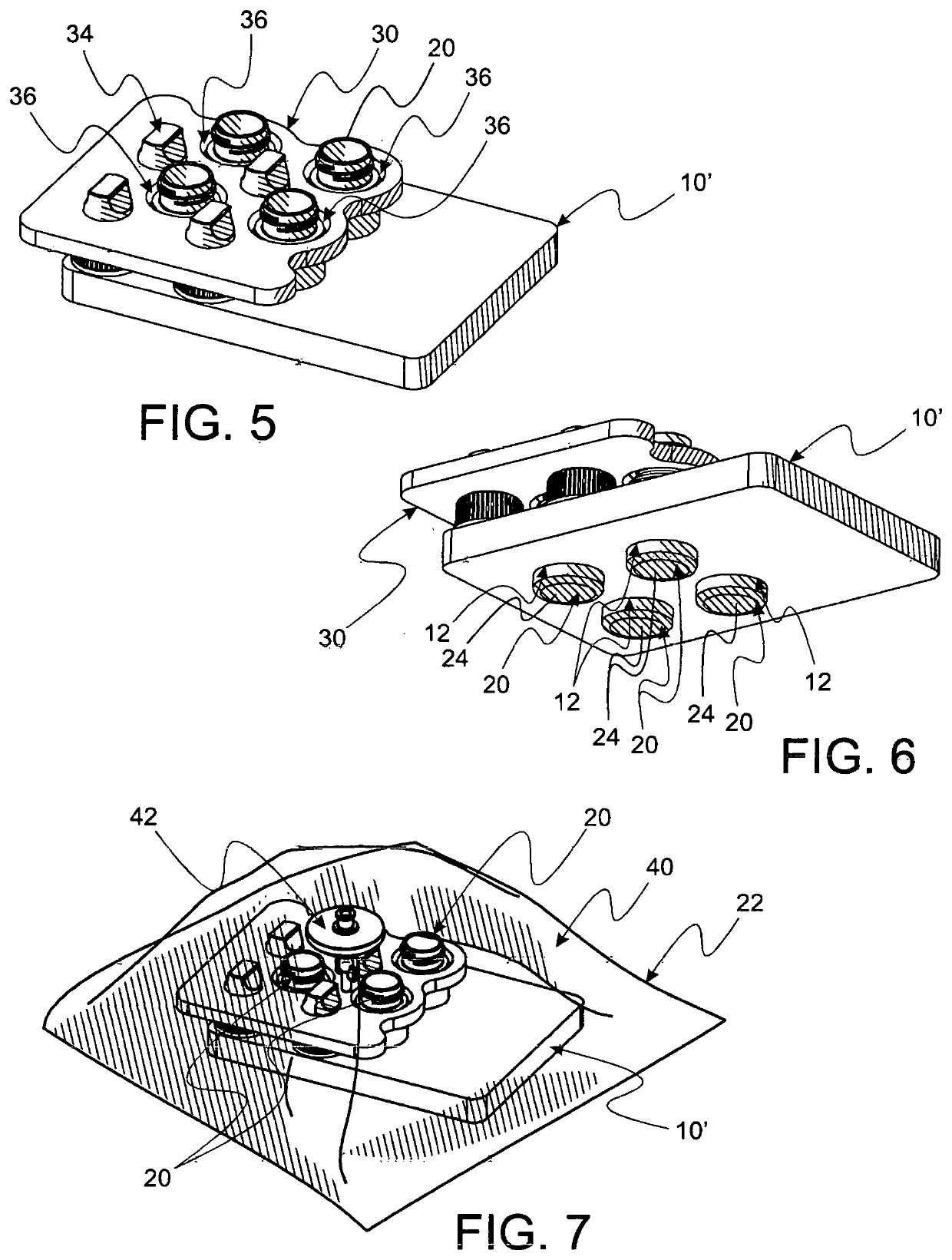
Method and apparatus for contamination-free transfer of a hazardous drug
A method for contamination-free transfer of liquid from one container to another, and devices to carry out the method are disclosed. Contamination-free transfer means that during the transfer process there is no (1) leakage of (a) the liquid, (b) air contaminated by the liquid, and (c) vapor from the liquid to the surroundings, and (2) surrounding contaminants from outside the containers that contact the liquid. The method's advantages include its simplicity, and the contamination-free transfer as defined above. The devices are adapted to effect contamination-free transfer of a hazardous drug to and from any container equipped with a standard connector port.
Owner:EQUASHIELD MEDICAL
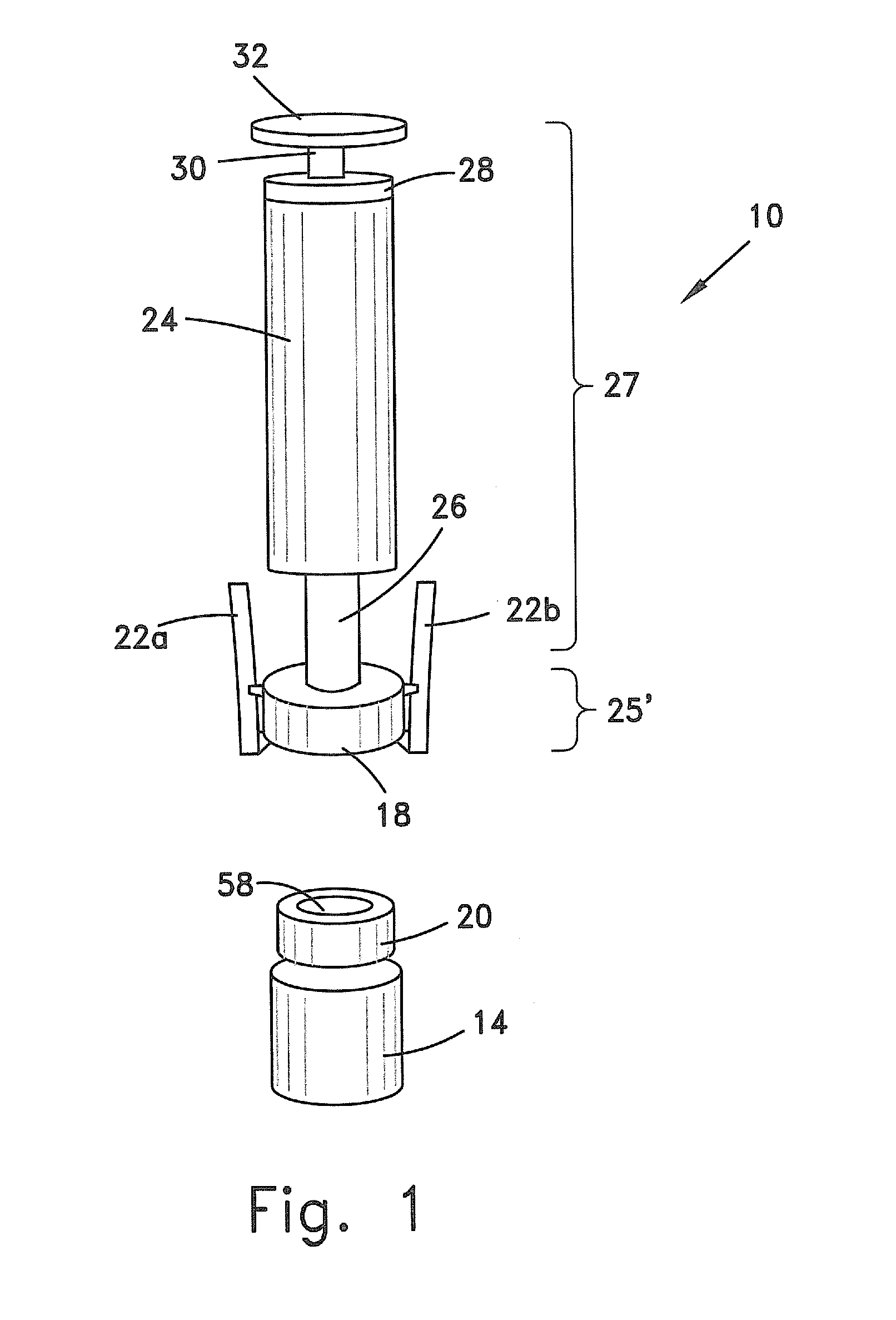
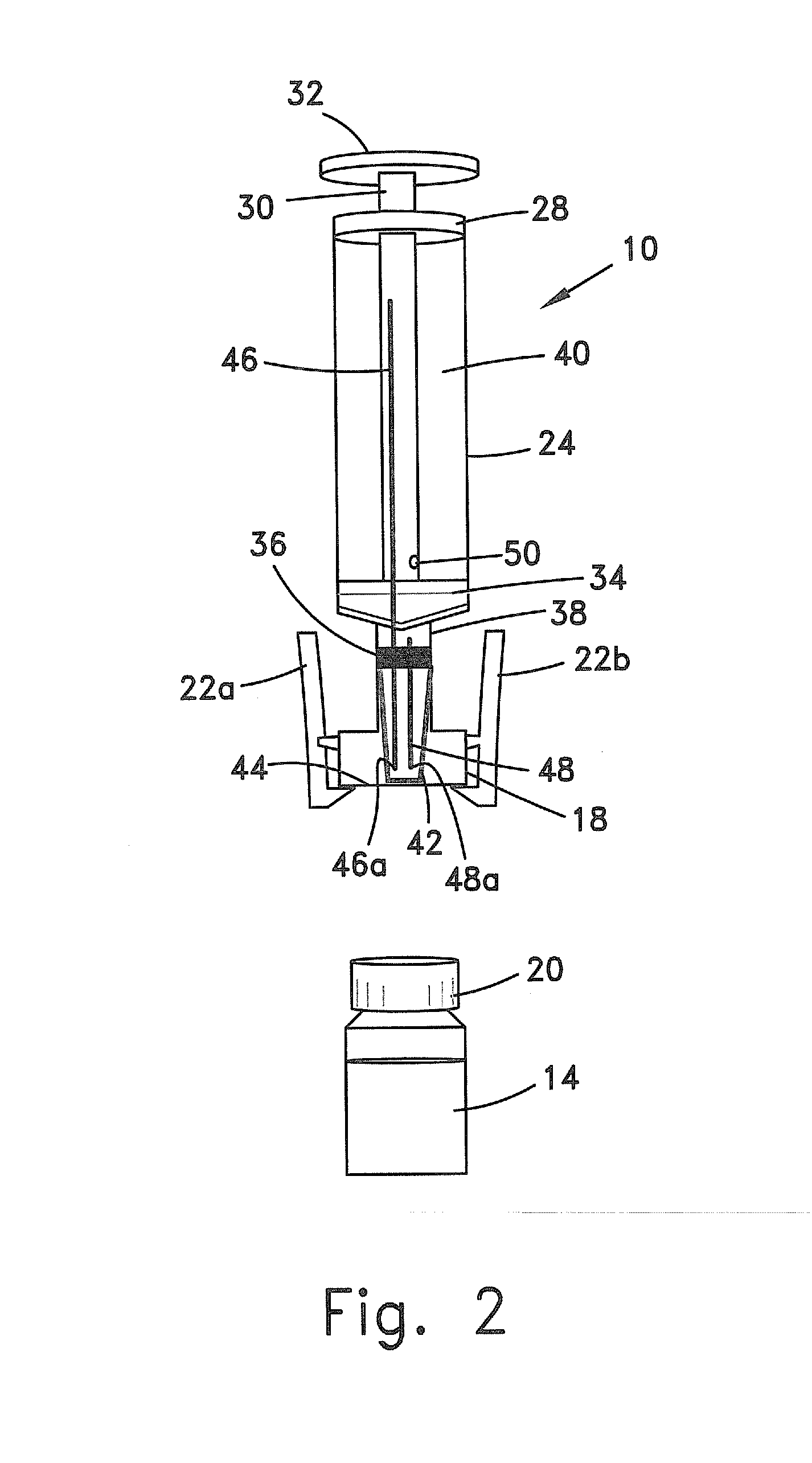
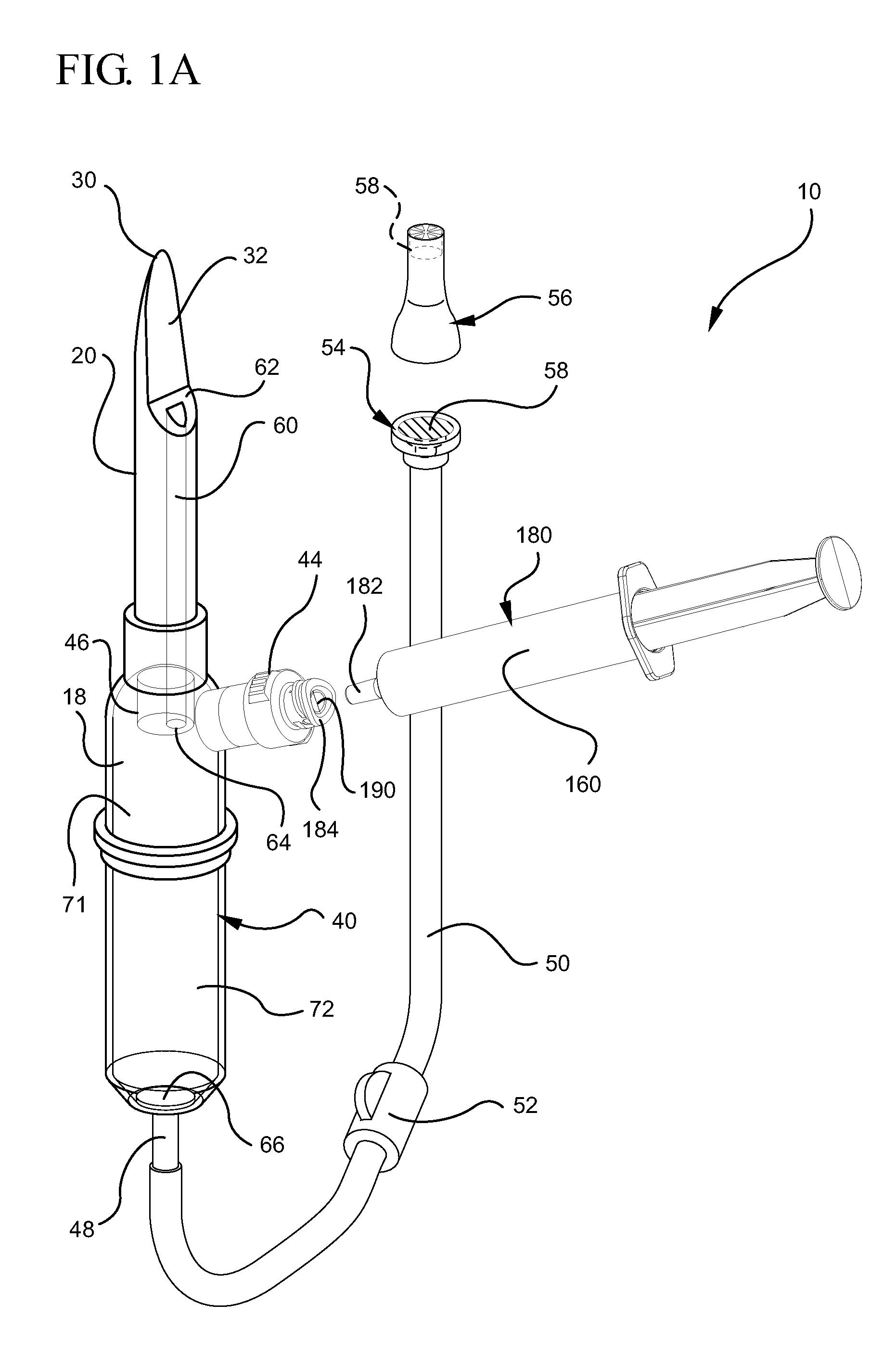
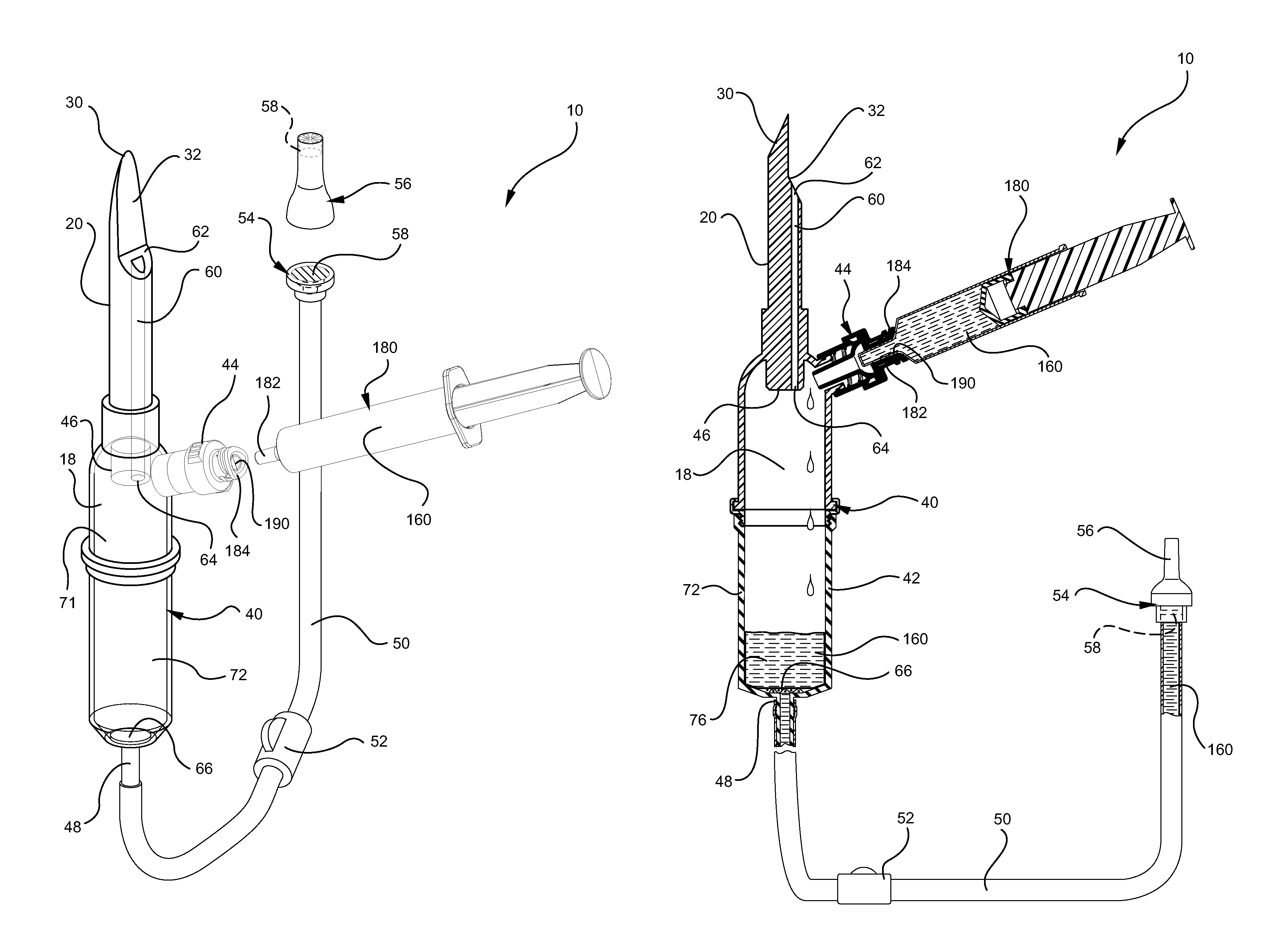
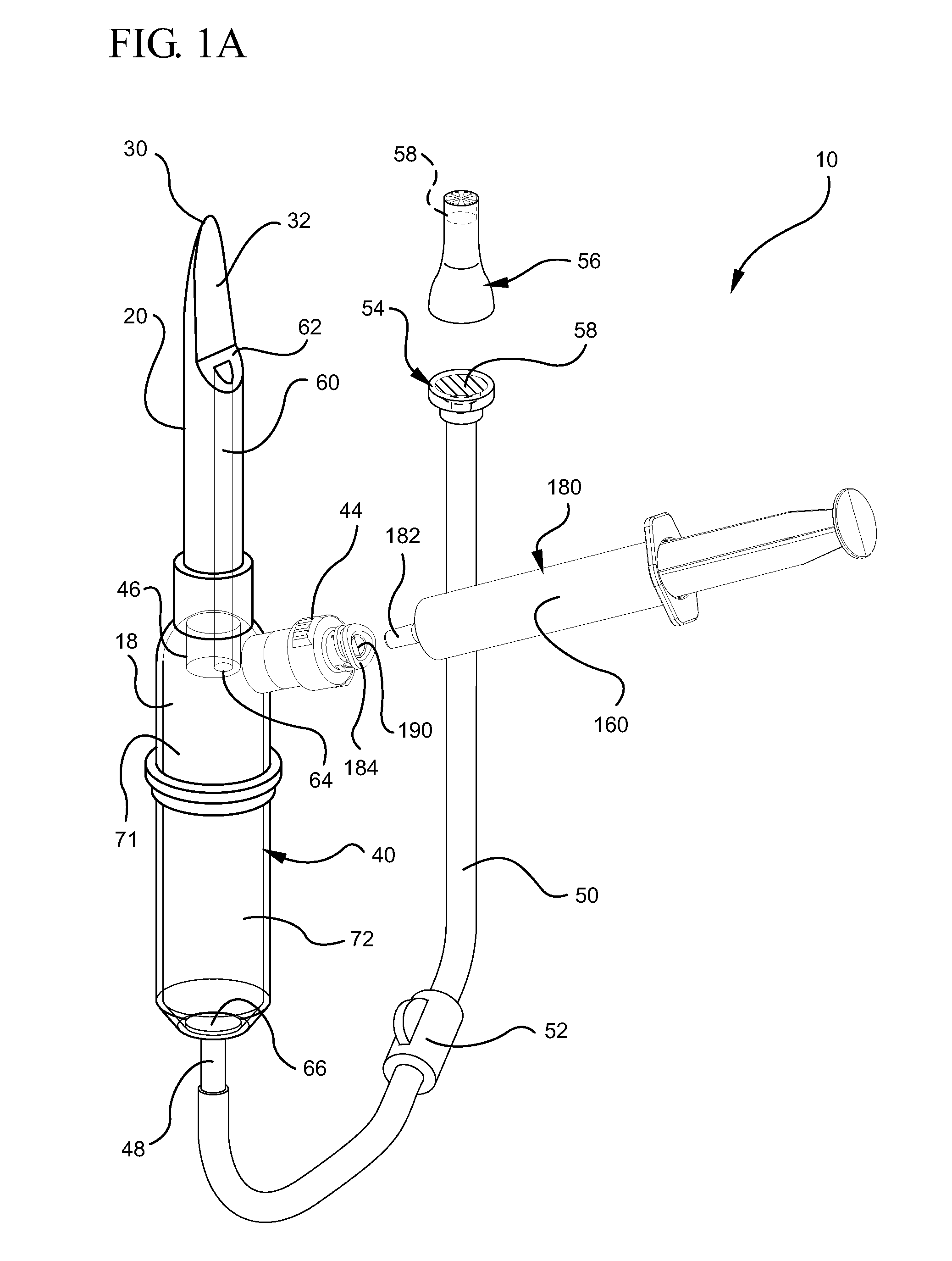
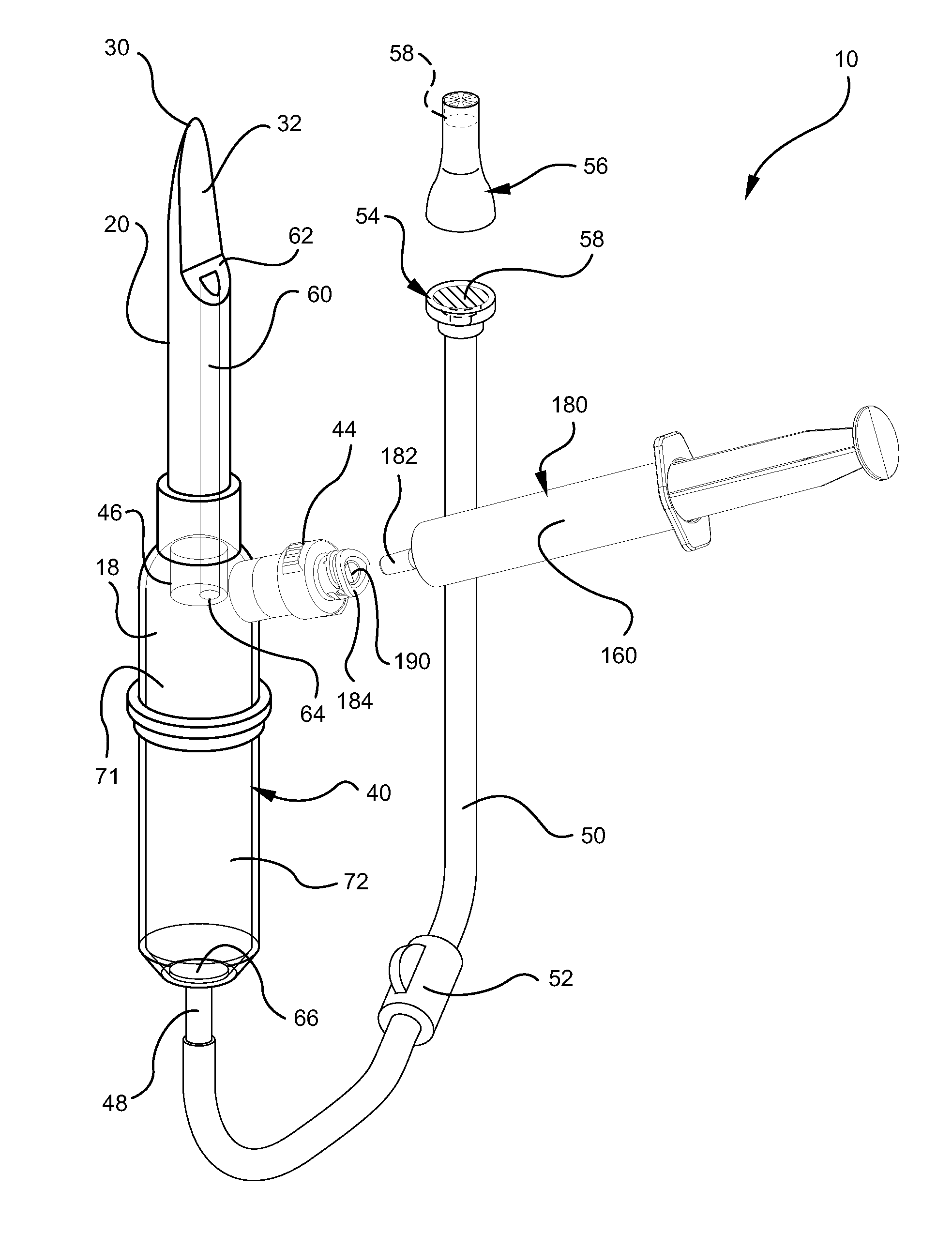
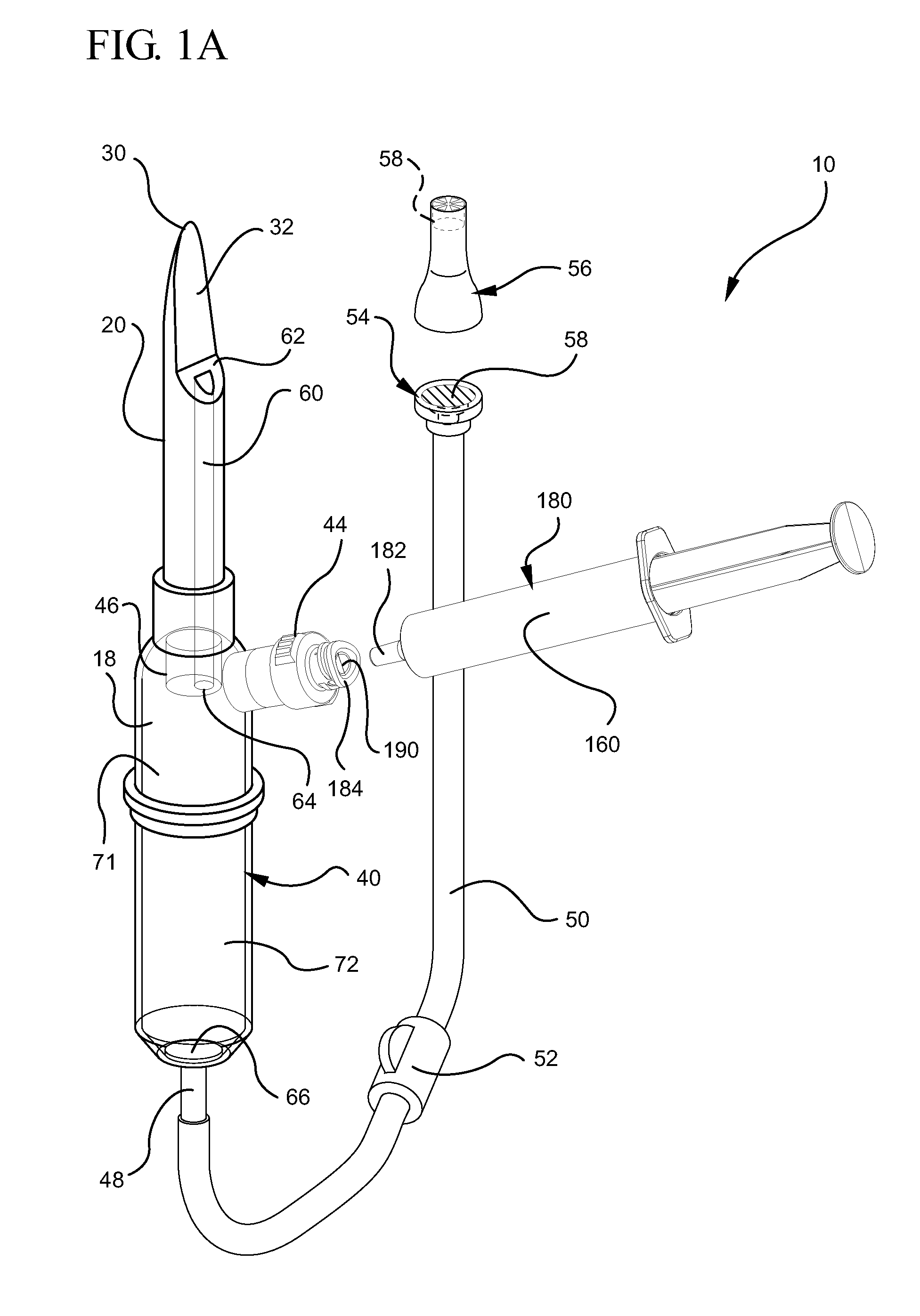
Systems and methods for providing a closed venting hazardous drug iv set
ActiveUS20110275988A1Avoid exposure to hazardsAvoid contactDiagnosticsSurgeryInfusion ProcedureHazardous drugs
A device for priming and venting a hazardous drug within an intravenous administration set. The device includes various access ports and fluid channels to permit direct injection of a hazardous drug into the fluid reservoir, while eliminating the possibility of undesirable exposure to the hazardous drug. The device further includes priming and flushing ports to enable flushing of a hazardous drug from the system following an infusion procedure.
Owner:BECTON DICKINSON & CO
Systems and methods for providing a closed venting hazardous drug iv set
InactiveUS20110276031A1Minimize exposureAvoid exposureFiltering accessoriesMedical devicesEngineeringHazardous drugs
A device for priming and venting a hazardous drug within an intravenous administration set. The device includes various access ports and fluid channels to permit direct injection of a hazardous drug into the fluid reservoir, while eliminating the possibility of undesirable exposure to the hazardous drug. The device further includes priming and flushing ports to enable flushing of a hazardous drug from the system following an infusion procedure.
Owner:BECTON DICKINSON & CO
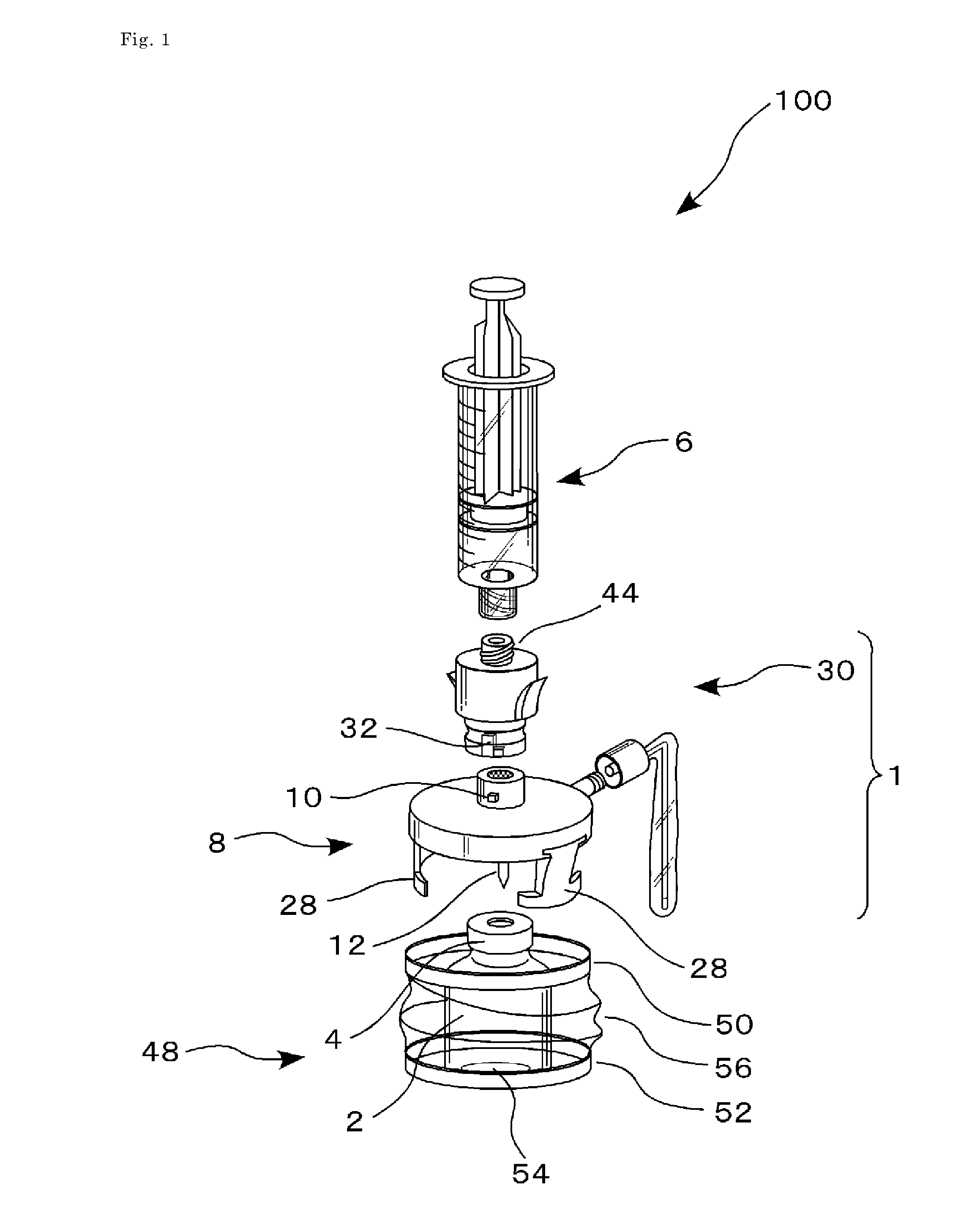
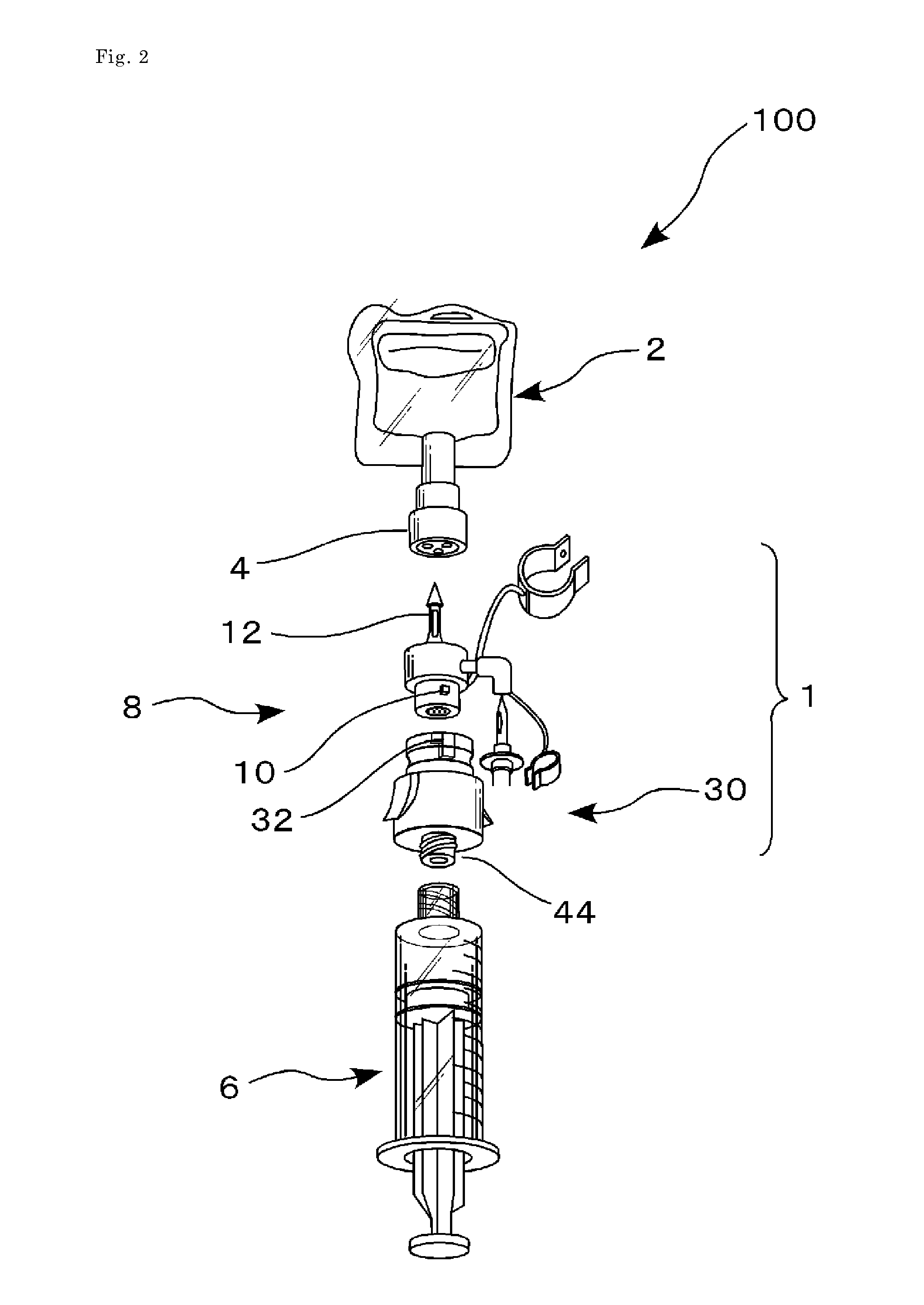
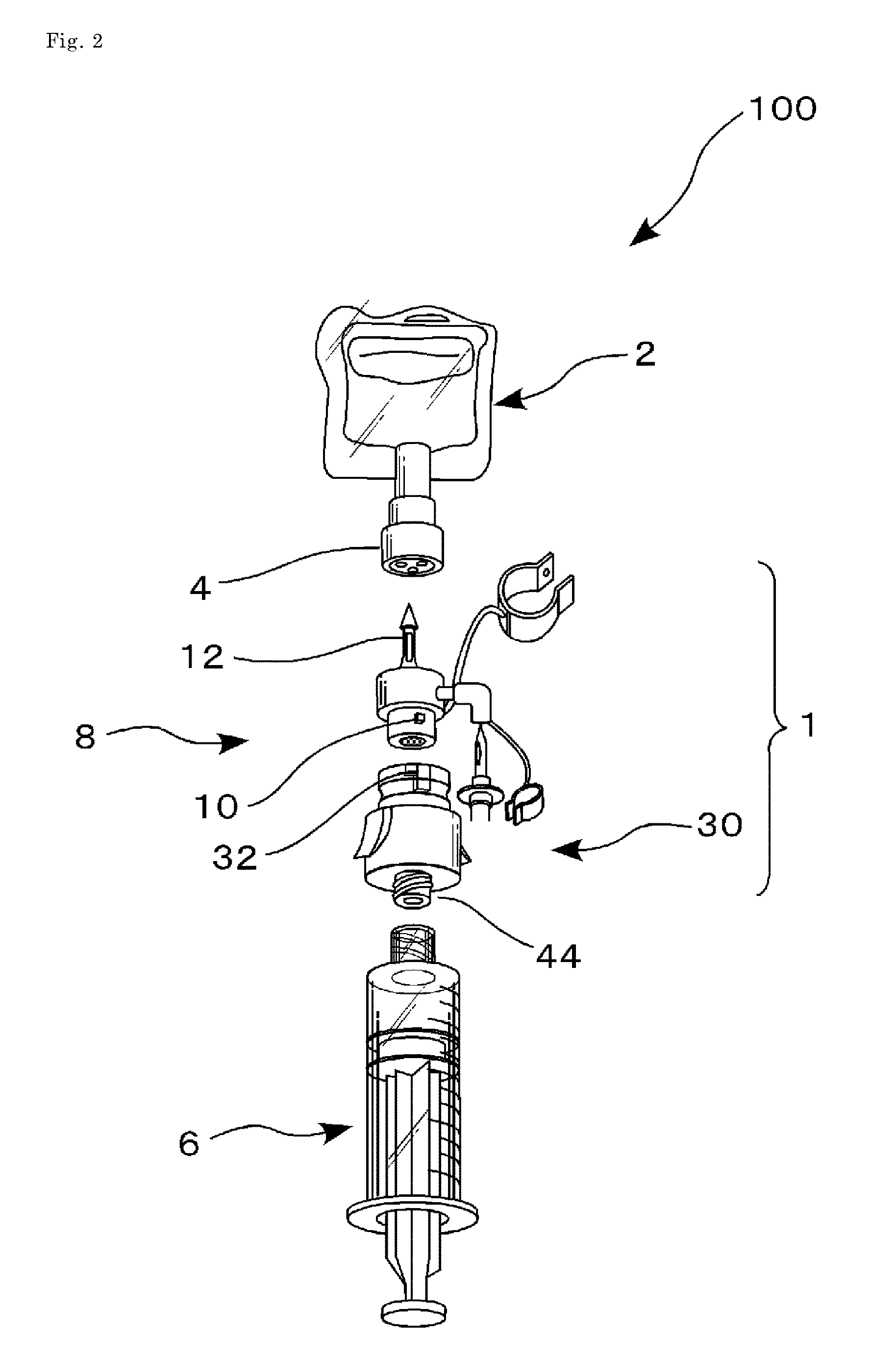
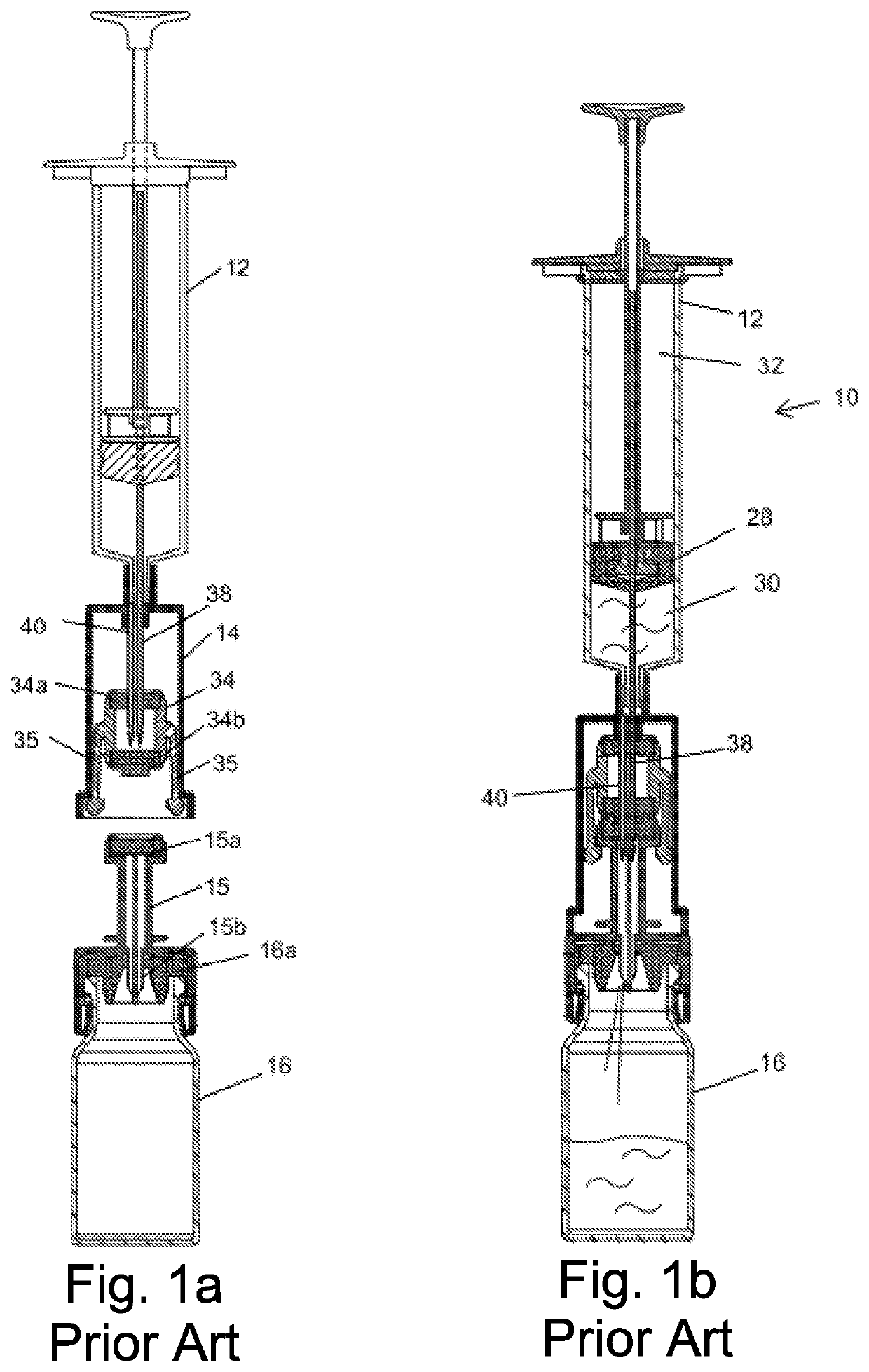
Drug delivery device
ActiveUS20150209233A1Avoid exposure to hazardsDiagnosticsSurgeryHazardous drugsBiomedical engineering
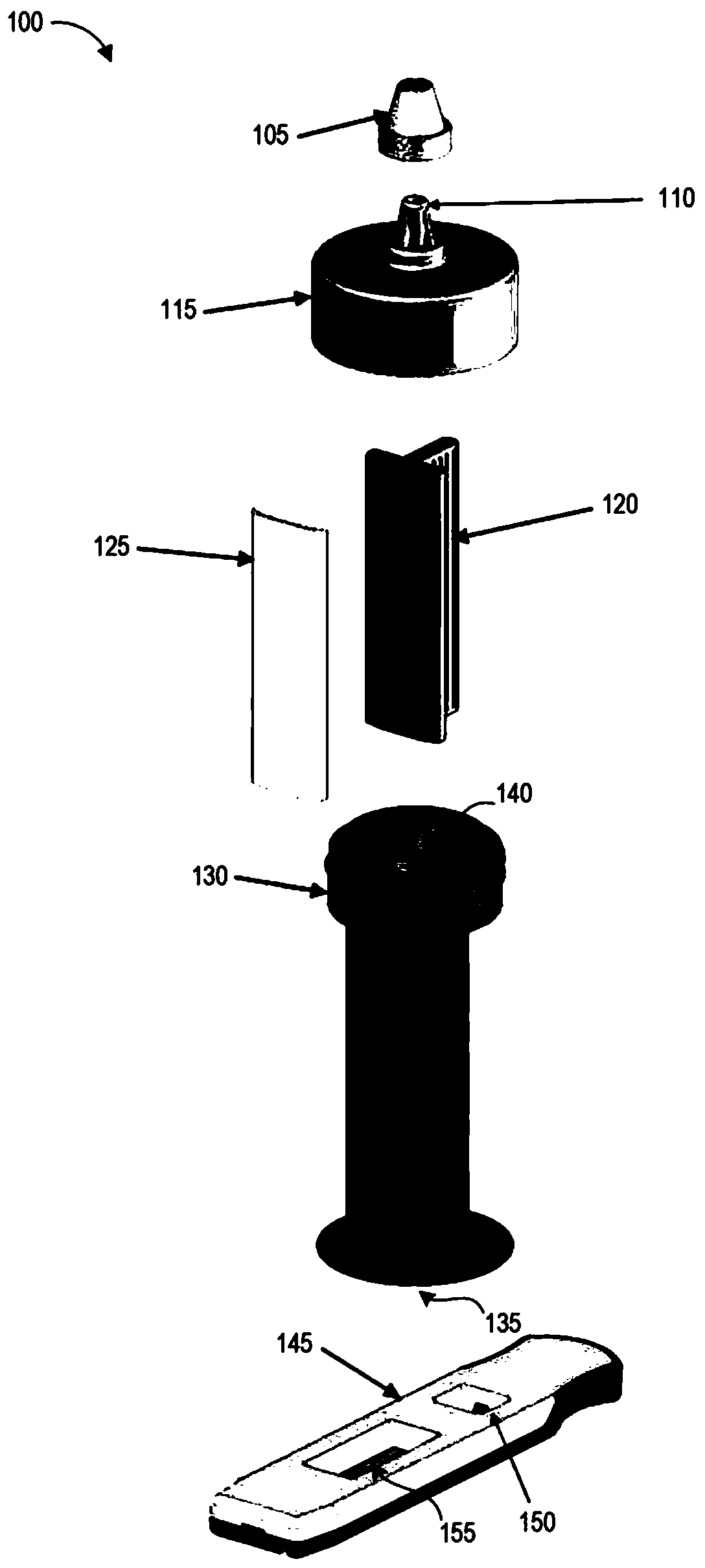
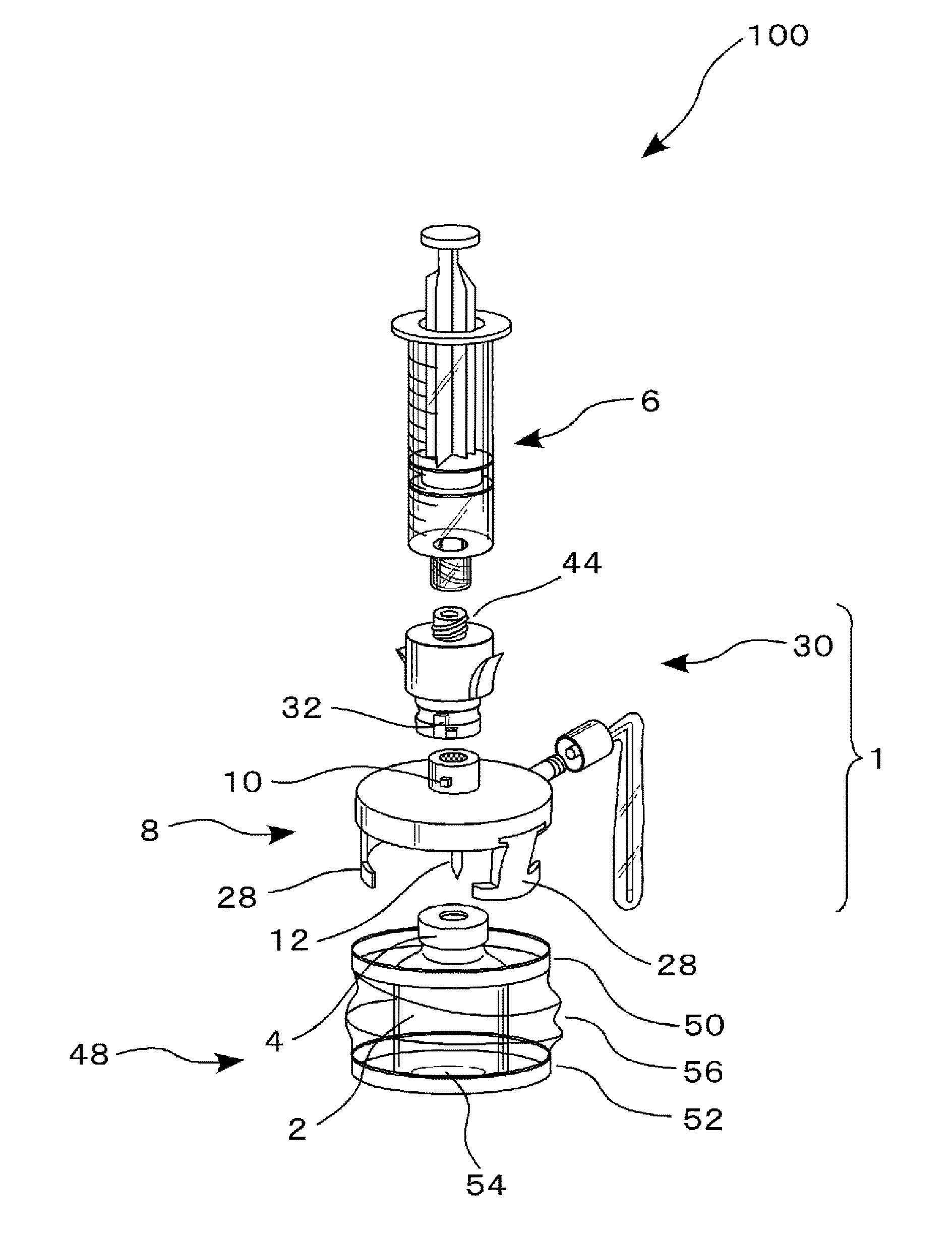
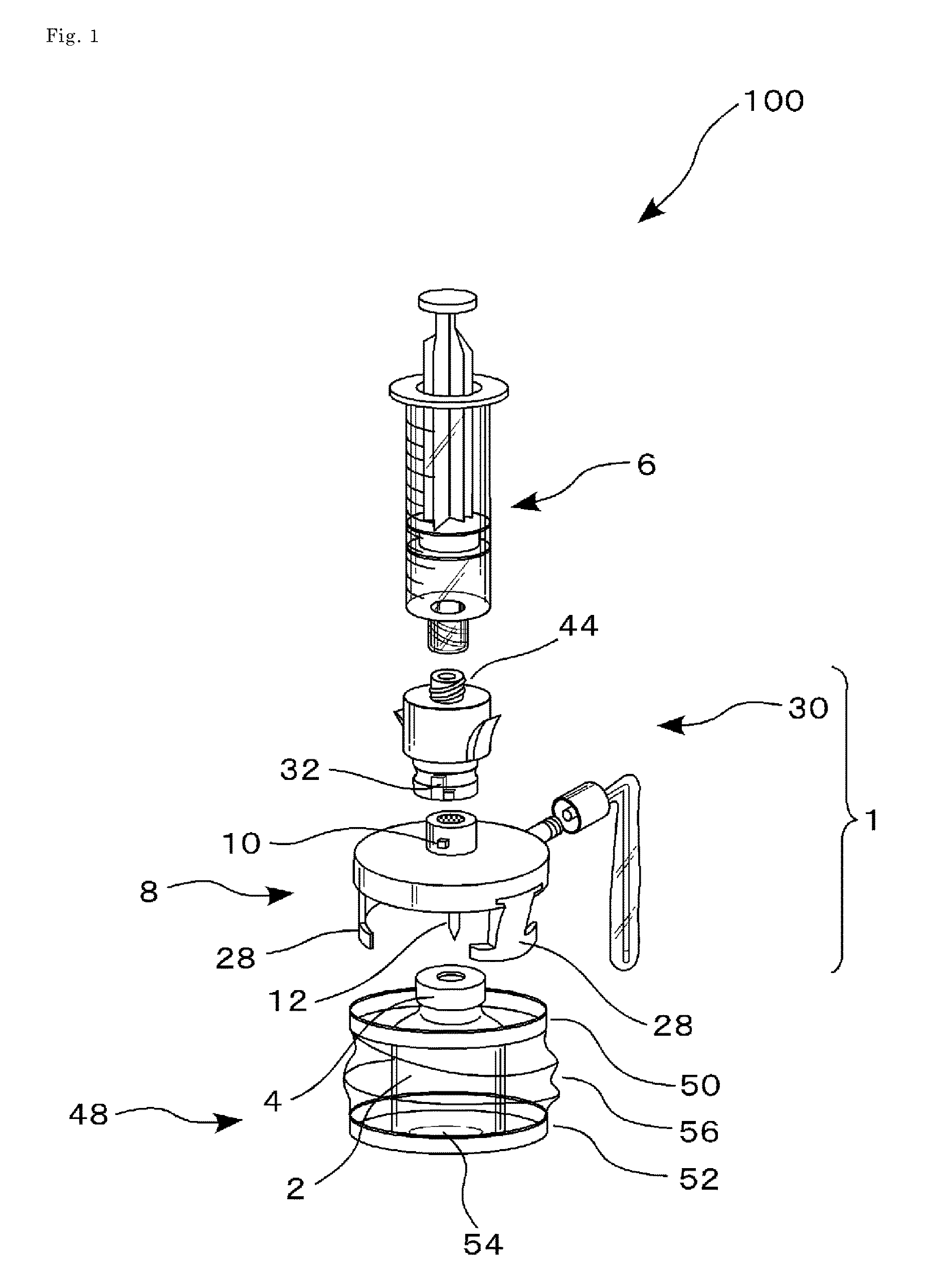
This drug delivery device delivers a drug between a syringe barrel and a drug container, and, by enveloping the entire the drug container used in drug delivery, prevents exposure to hazardous drugs adhering to the drug container. This drug delivery device includes: a first member connected to the drug container on the side of a stopper; a second member connected to the first member and to the syringe barrel; and a third member connected to the first member and covering the drug container in a sealed state. The first member has a first hollow needle member and a drug container holder, and the second member has a second hollow needle member. The third member has an opening portion, a bottom surface portion where the drug container is stably mounted, and a flexible member which is connected to the opening portion and the bottom surface portion.
Owner:KOBAYASHI & CO LTD
Method and apparatus for contamination-free transfer of a hazardous drug
The invention is a method that allows contamination-free transfer of a liquid from one container to another and devices including embodiments of a transfer apparatus and adaptors that are used to carry out the method. By contamination-free transfer of liquid it is meant that during the transfer process there is no leakage of the liquid or air contaminated by the liquid or vapors of the liquid to the surroundings and also that no contaminants from the surroundings come into contact with the liquid. The main advantages of the method, in addition to its simplicity, is that at no stage of the transfer procedure is there leakage of the liquid or air contaminated by the liquid or vapors of the liquid to the surroundings and also that no contaminants from the surroundings come into contact with the liquid. The present invention is particularly directed towards providing an apparatus that is adapted to effect contamination-free transfer of a hazardous drug to and from any container equipped with a standard connector port.
Owner:EQUASHIELD MEDICAL
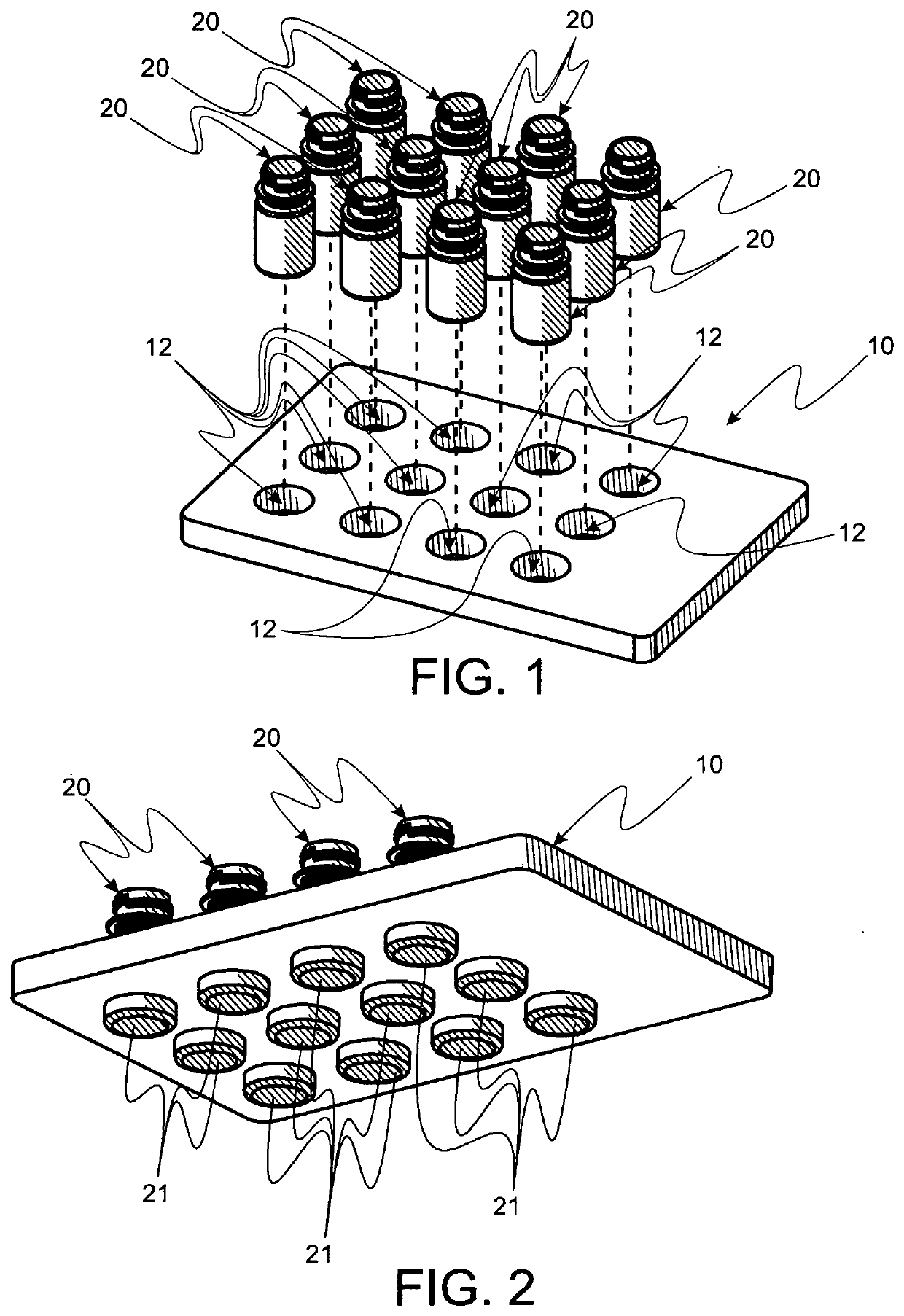
Vial transfer convenience IV kits and methods
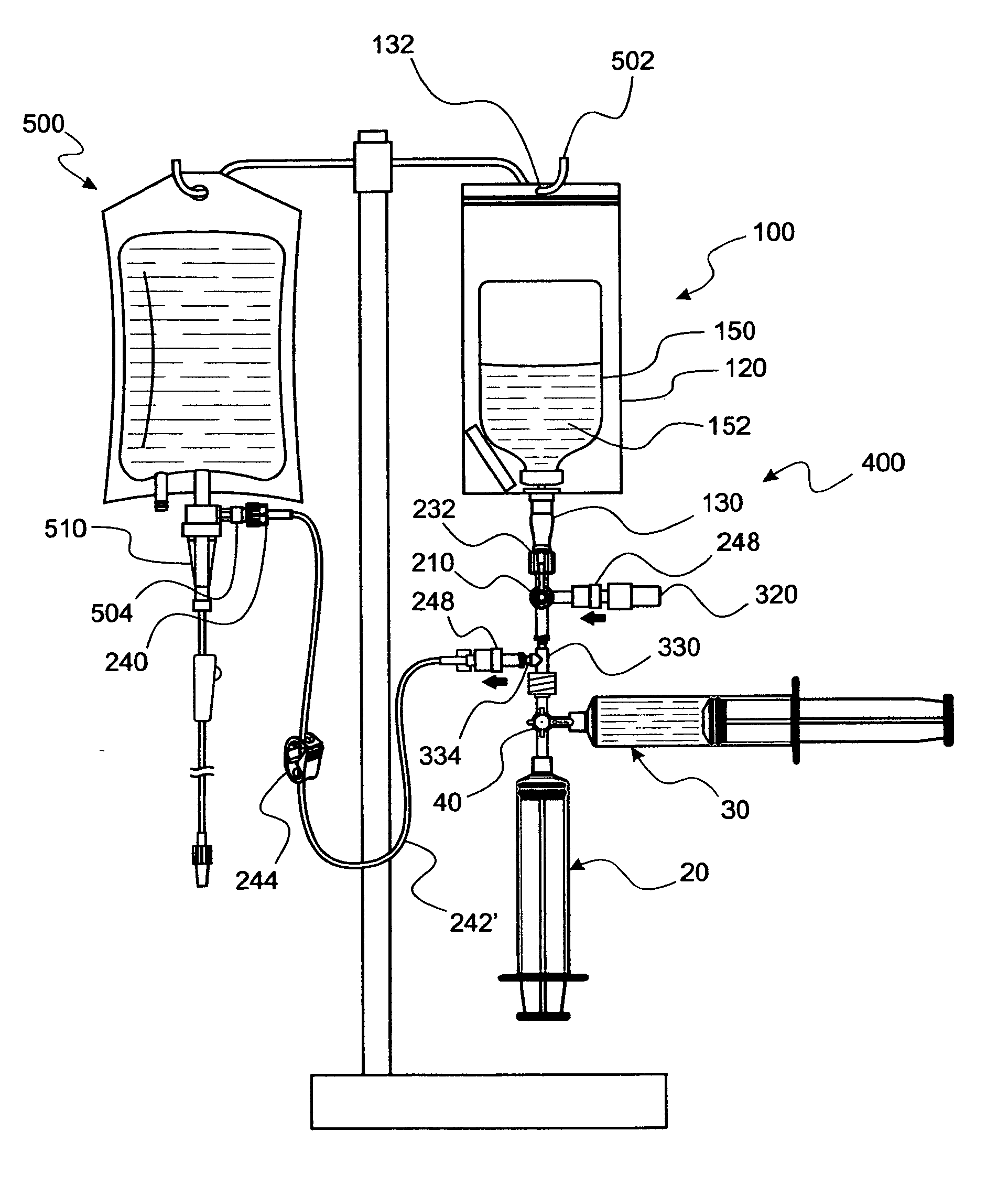
InactiveUS20090306621A1Reduce disconnectionImprove securityInfusion devicesPharmaceutical containersEngineeringIntravenous therapy
Convenience kits designed to provide for closed, but selectable liquid transfer from a vial to a variety of IV containers and medical syringes. In particular, a kit for fully enclosing a vial for safety in hazardous drug transfer is disclosed. Generally, the kits contain unitized parts wherever reasonable to limit makes and breaks. Further, pathway determining kits provide selectable pathways for purging connections wish flushing solution where makes and breaks are made between various fluid pathway involved parts such that, when disconnections are made, flush solution is resident at the exposed interface. Also disclosed is a 3-way valve as part of a closed, switchable pathway controlling subsystem by which pathways are selected for reconstituting dry medicine in a vial, displacing a measured dose of liquid from a vial, exchanging gas into the vial for displaced liquid, delivering the measured dose to an IV container.
Owner:INTRAVENA
Systems and methods for providing a closed venting hazardous drug IV set
Owner:BECTON DICKINSON & CO
Systems and methods for providing a closed venting hazardous drug iv set
InactiveUS20110276010A1Avoid exposure to hazardsAvoid contactFiltering accessoriesMedical devicesEngineeringHazardous drugs
A device for priming and venting a hazardous drug within an intravenous administration set. The device includes various access ports and fluid channels to permit direct injection of a hazardous drug into the fluid reservoir, while eliminating the possibility of undesirable exposure to the hazardous drug. The device further includes priming and flushing ports to enable flushing of a hazardous drug from the system following an infusion procedure.
Owner:BECTON DICKINSON & CO
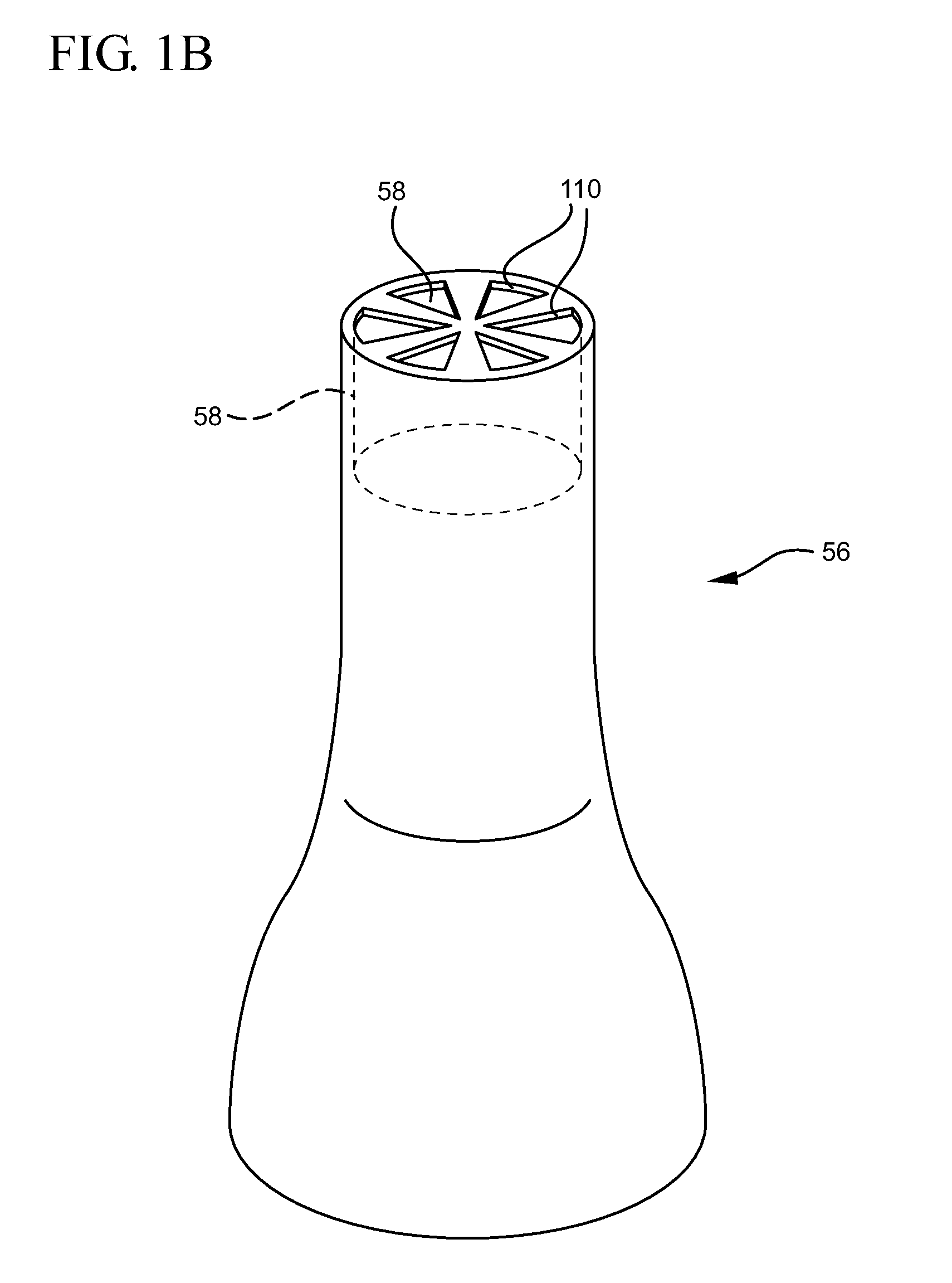
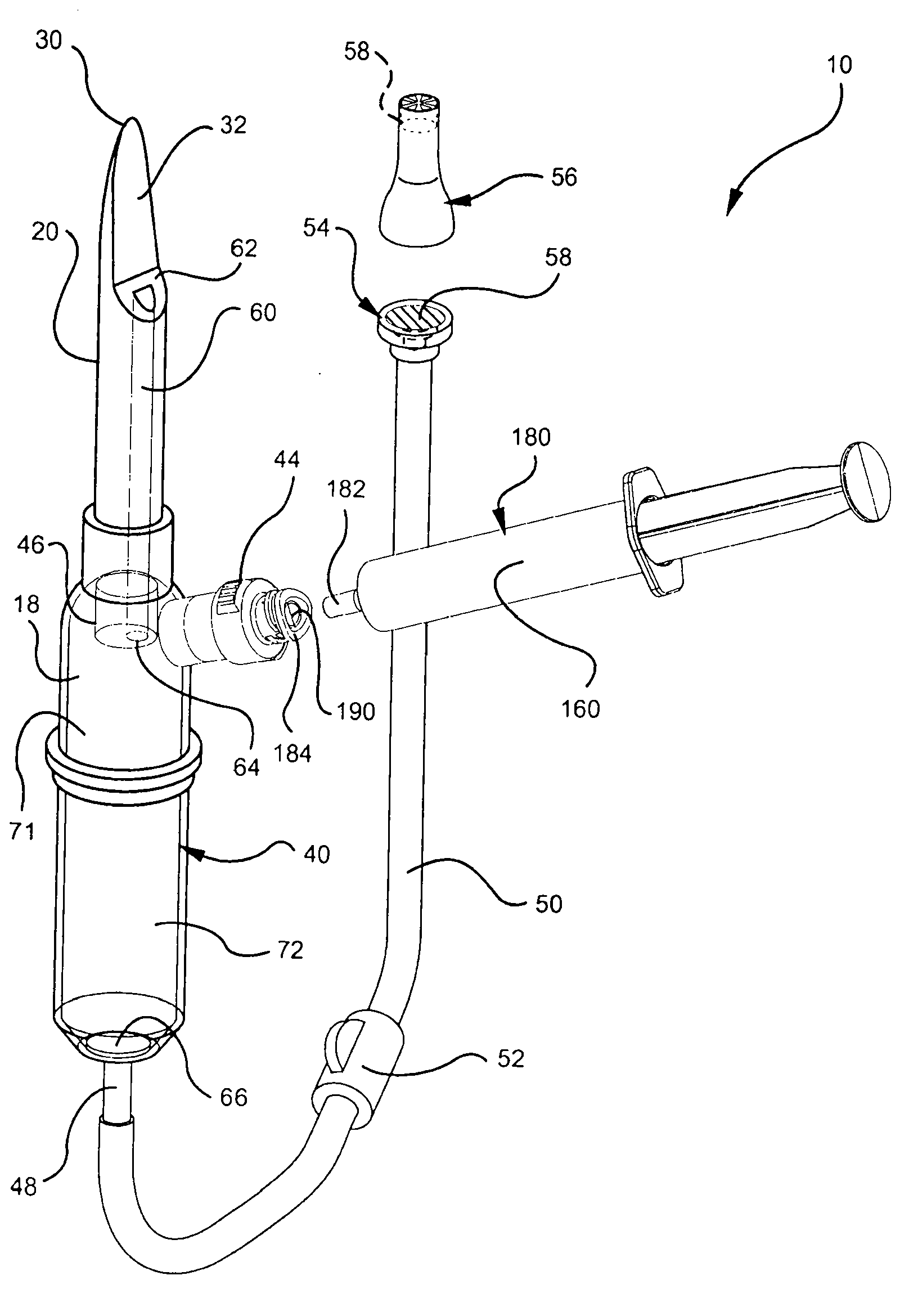
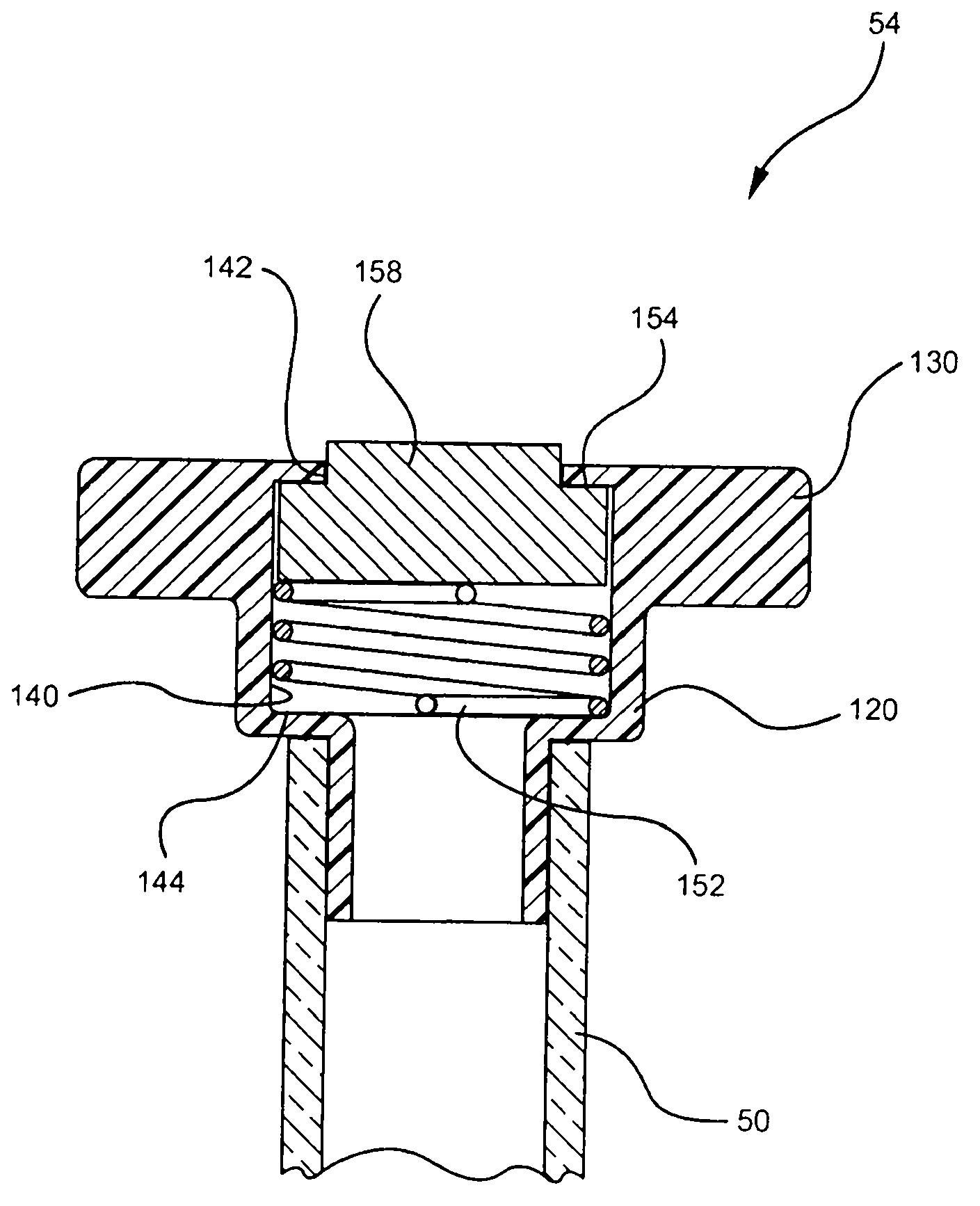
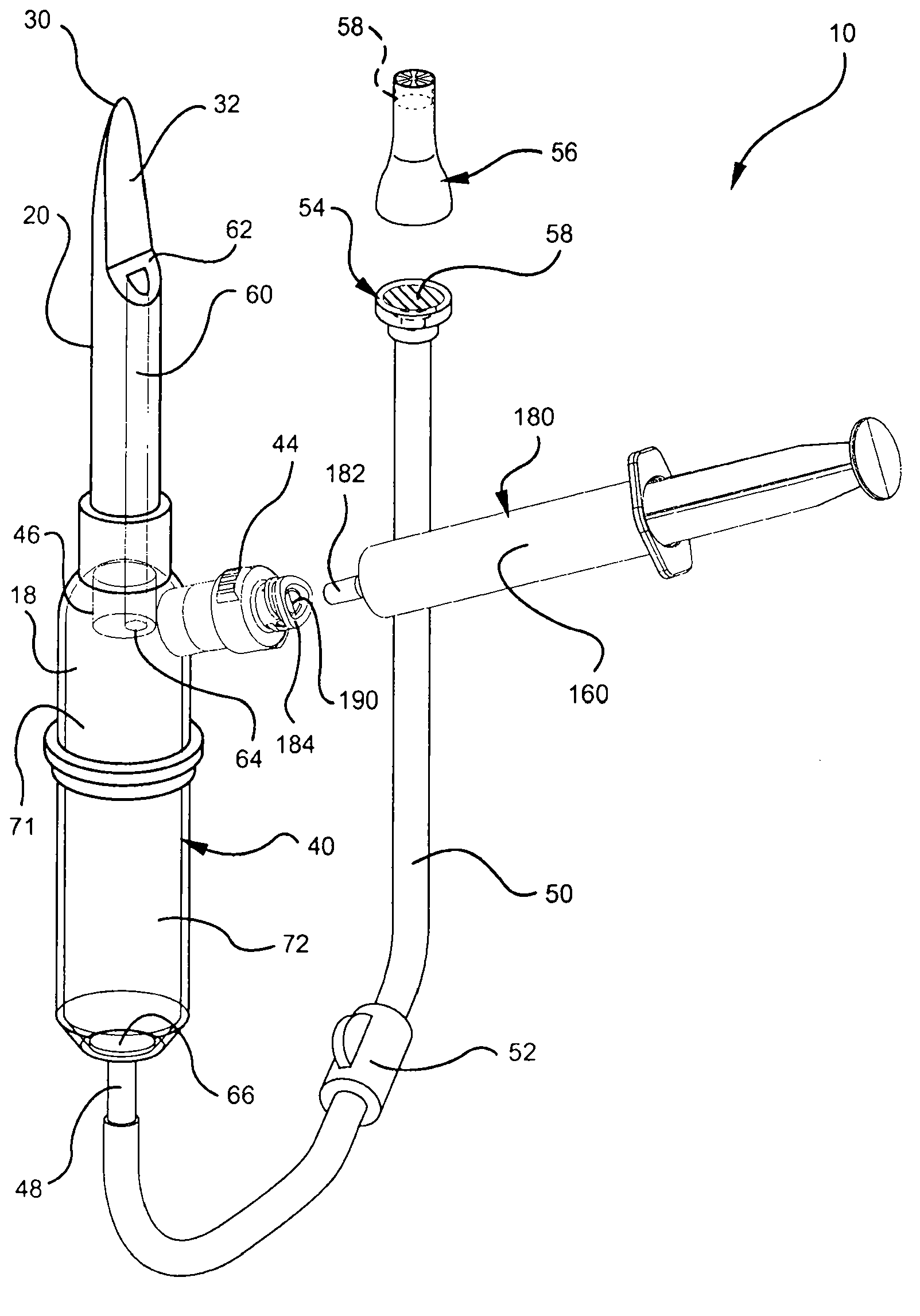
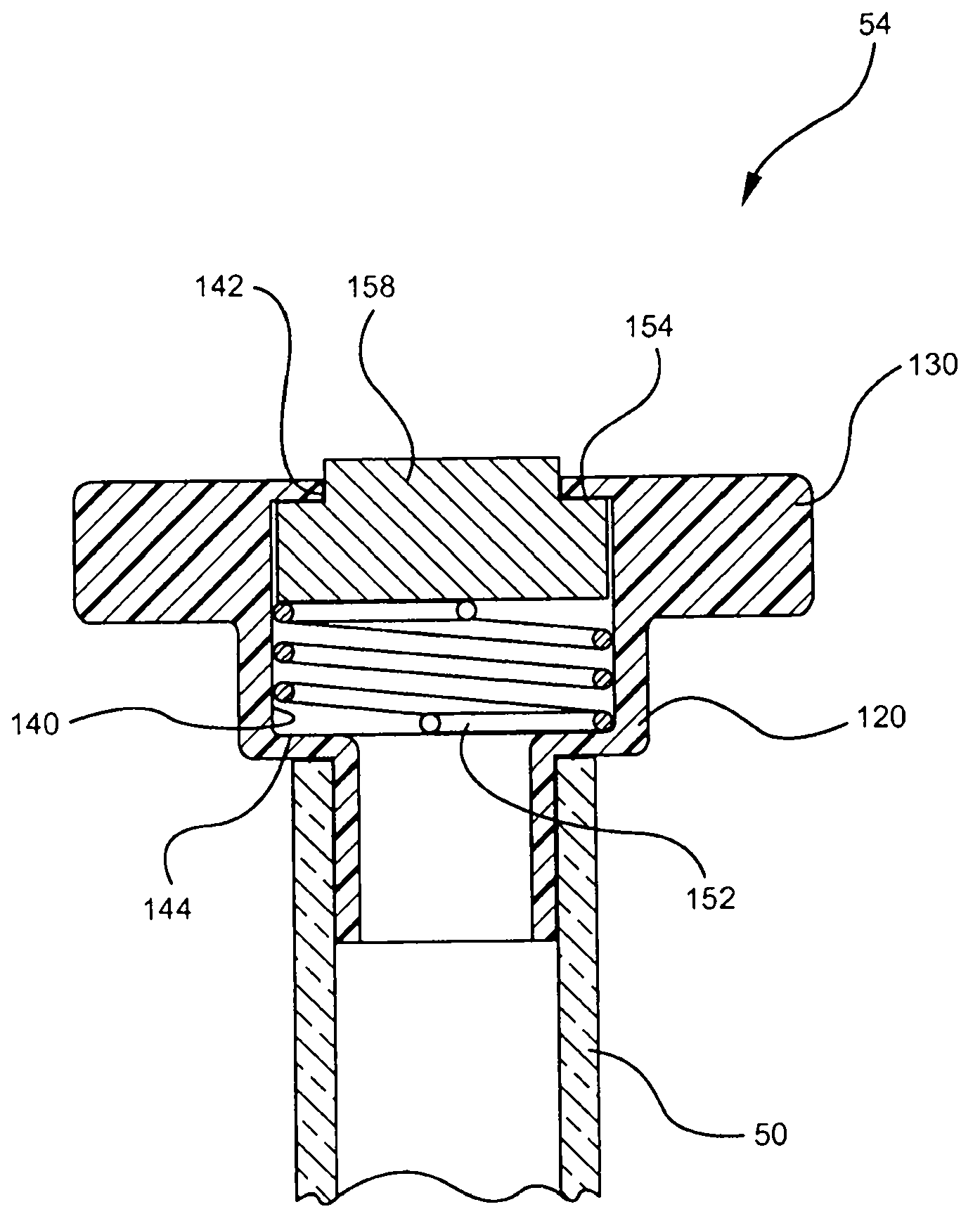
Systems and methods for providing a closed venting hazardous drug IV set
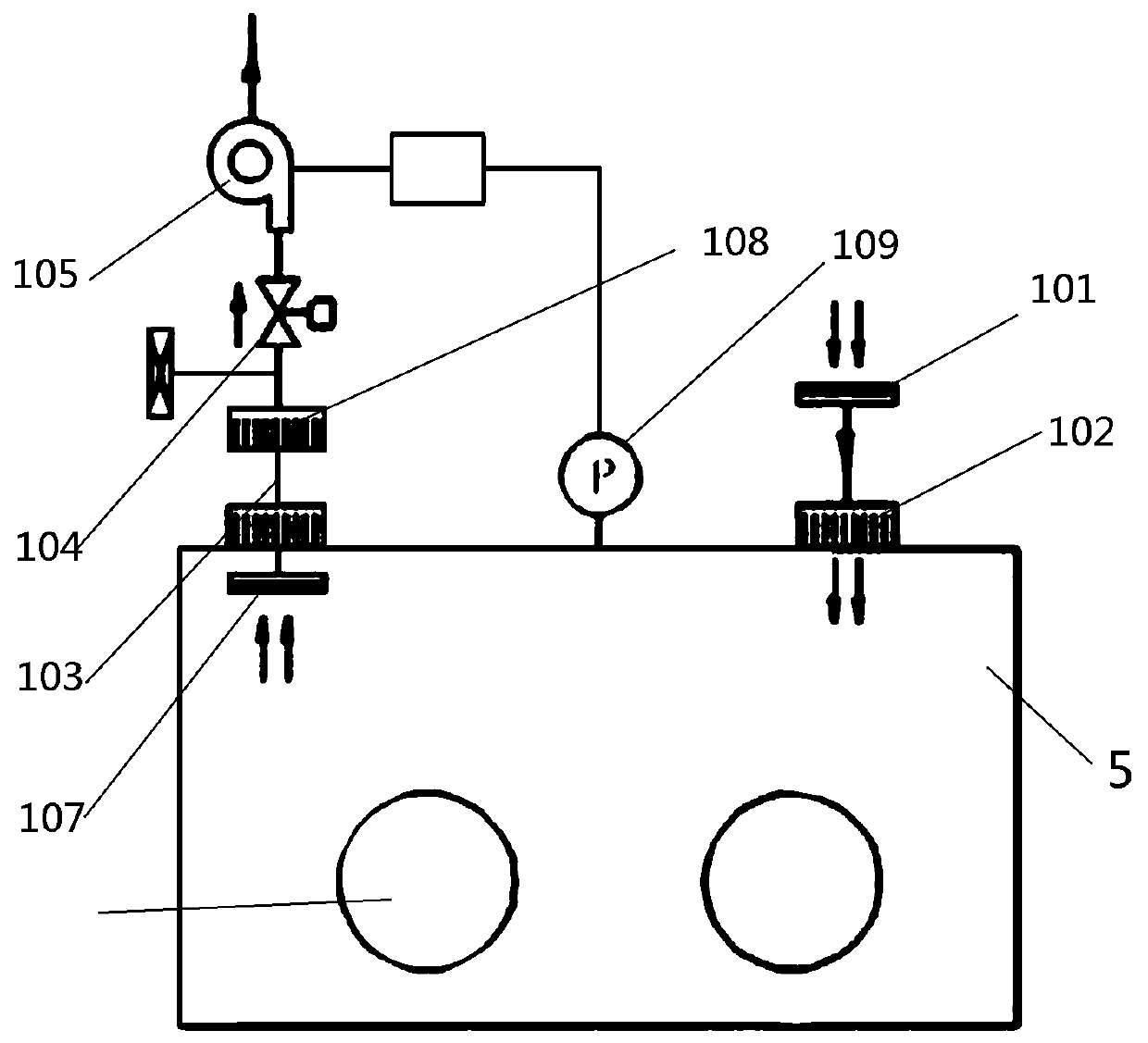
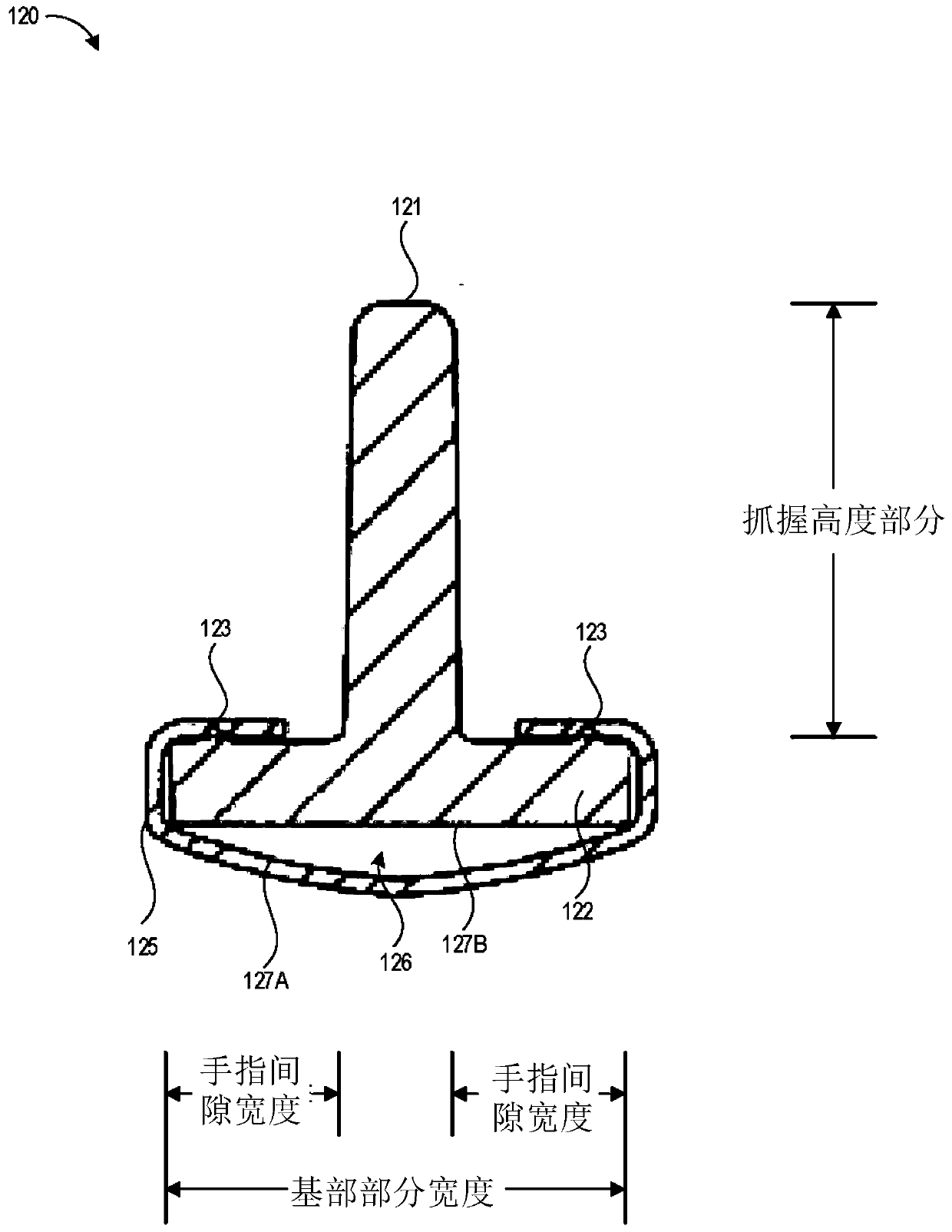
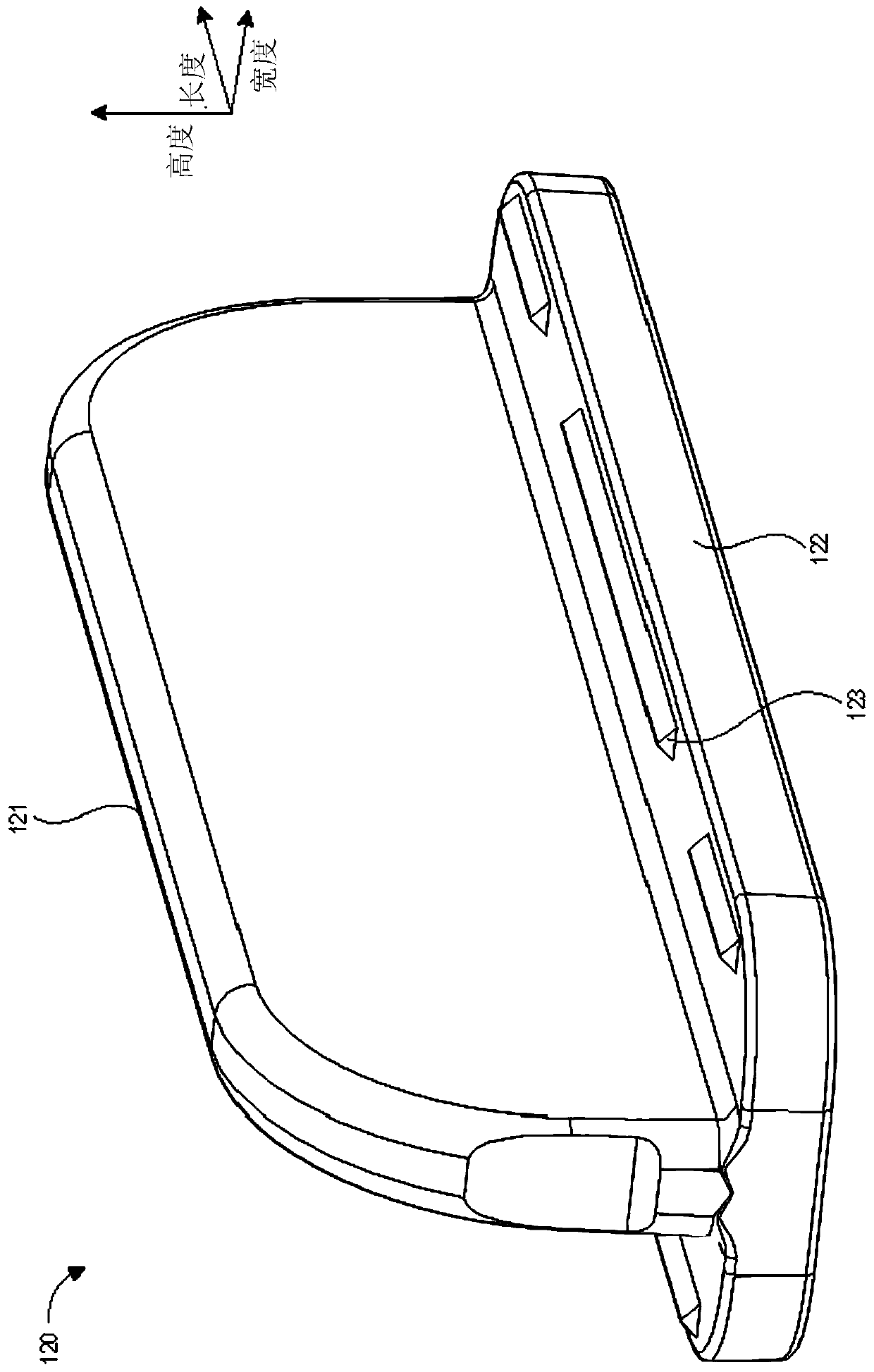
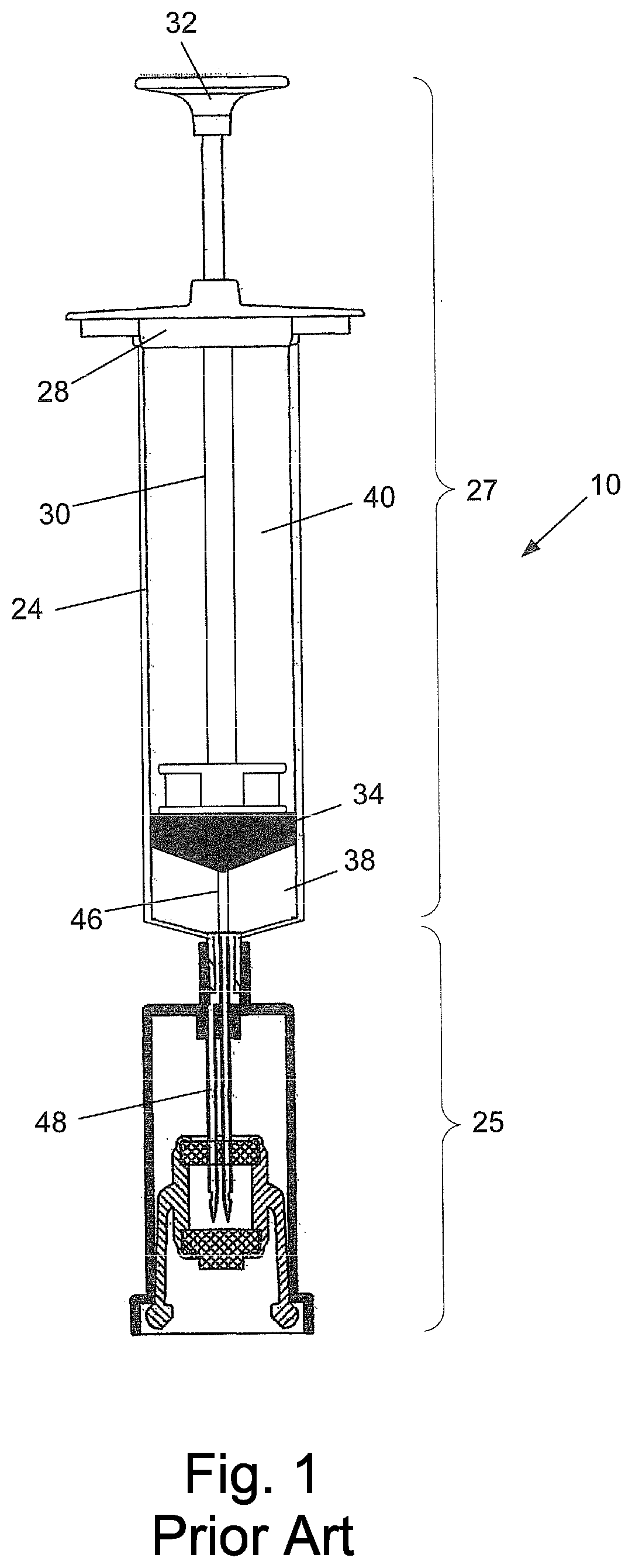
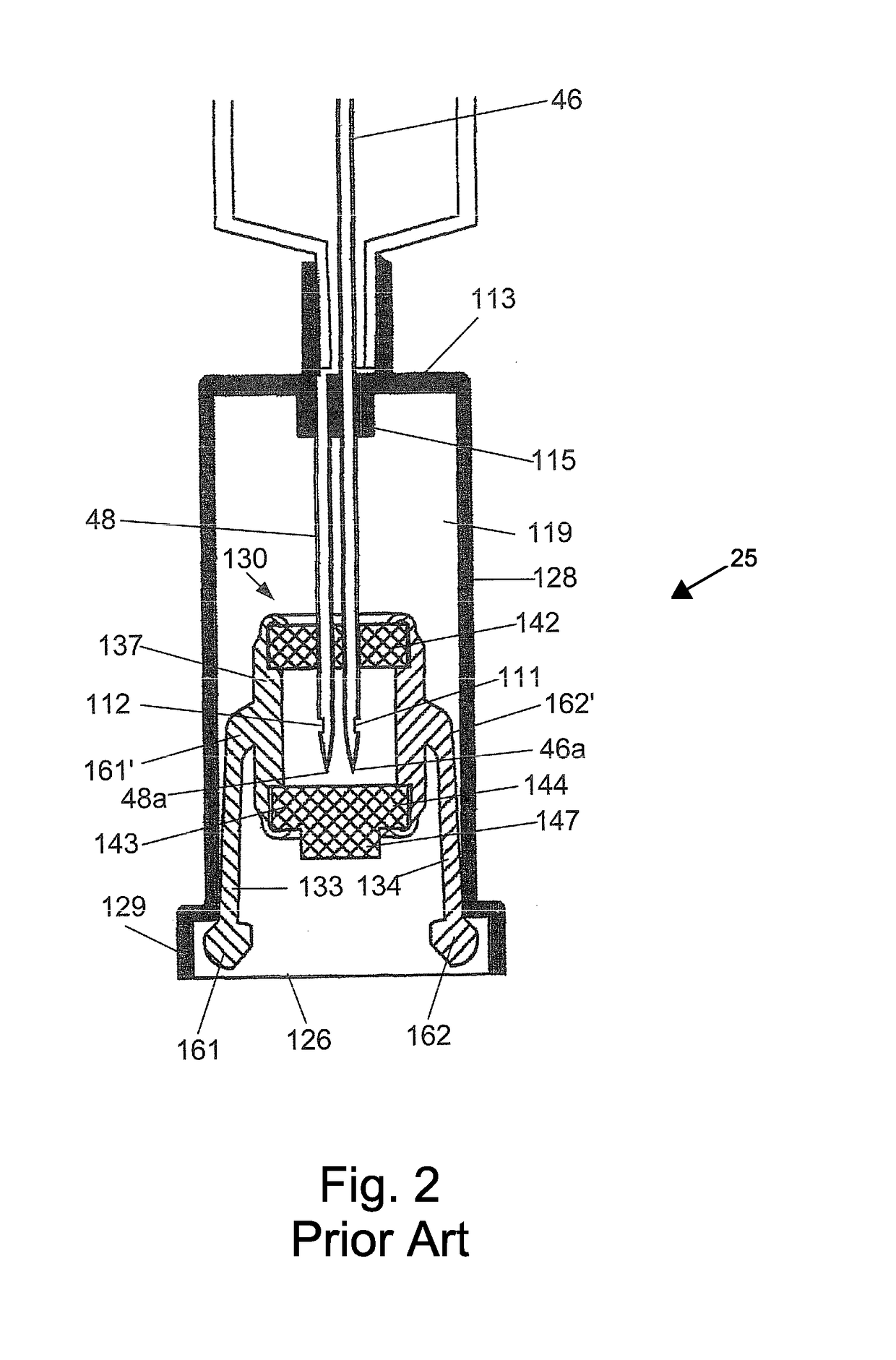
A device for priming and venting a hazardous drug within an intravenous administration set (10). The device includes various access ports (44, 28, 86) and fluid channels (50, 150) to permit direct injection of a hazardous drug into the fluid reservoir (24), while eliminating the possibility of undesirable exposure to the hazardous drug. The device further includes priming and flushing ports to enable flushing of a hazardous drug from the system following an infusion procedure.
Owner:BECTON DICKINSON & CO
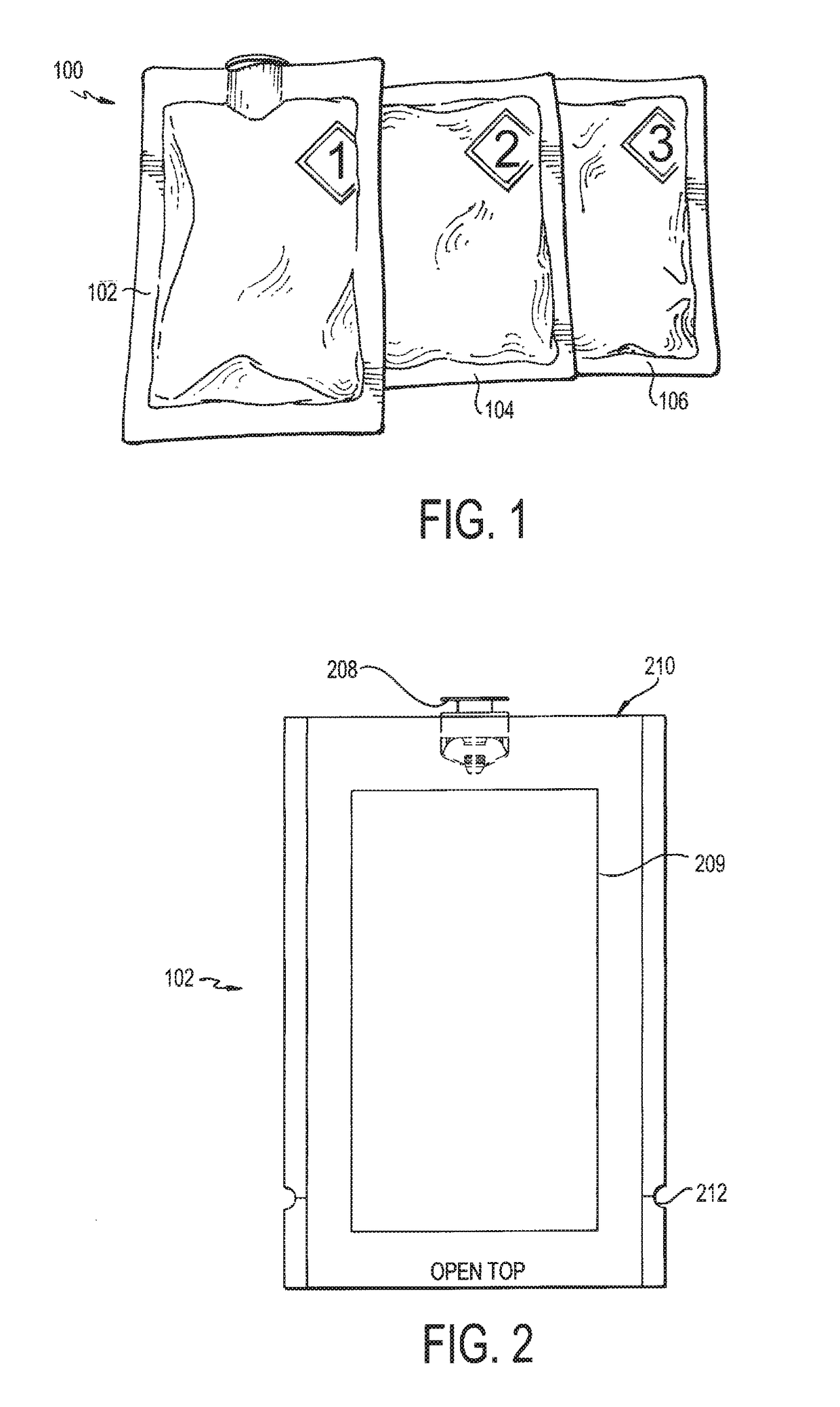
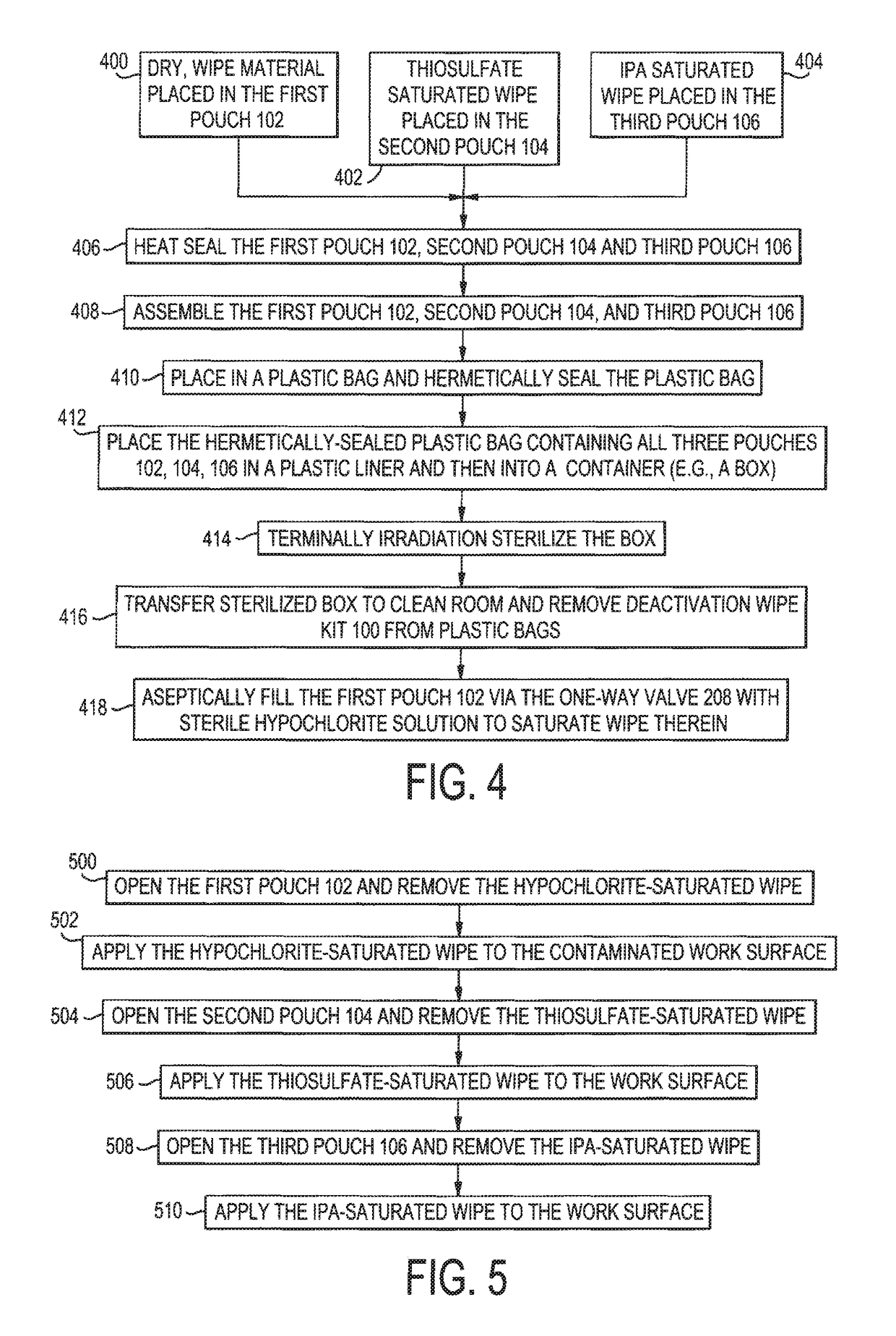
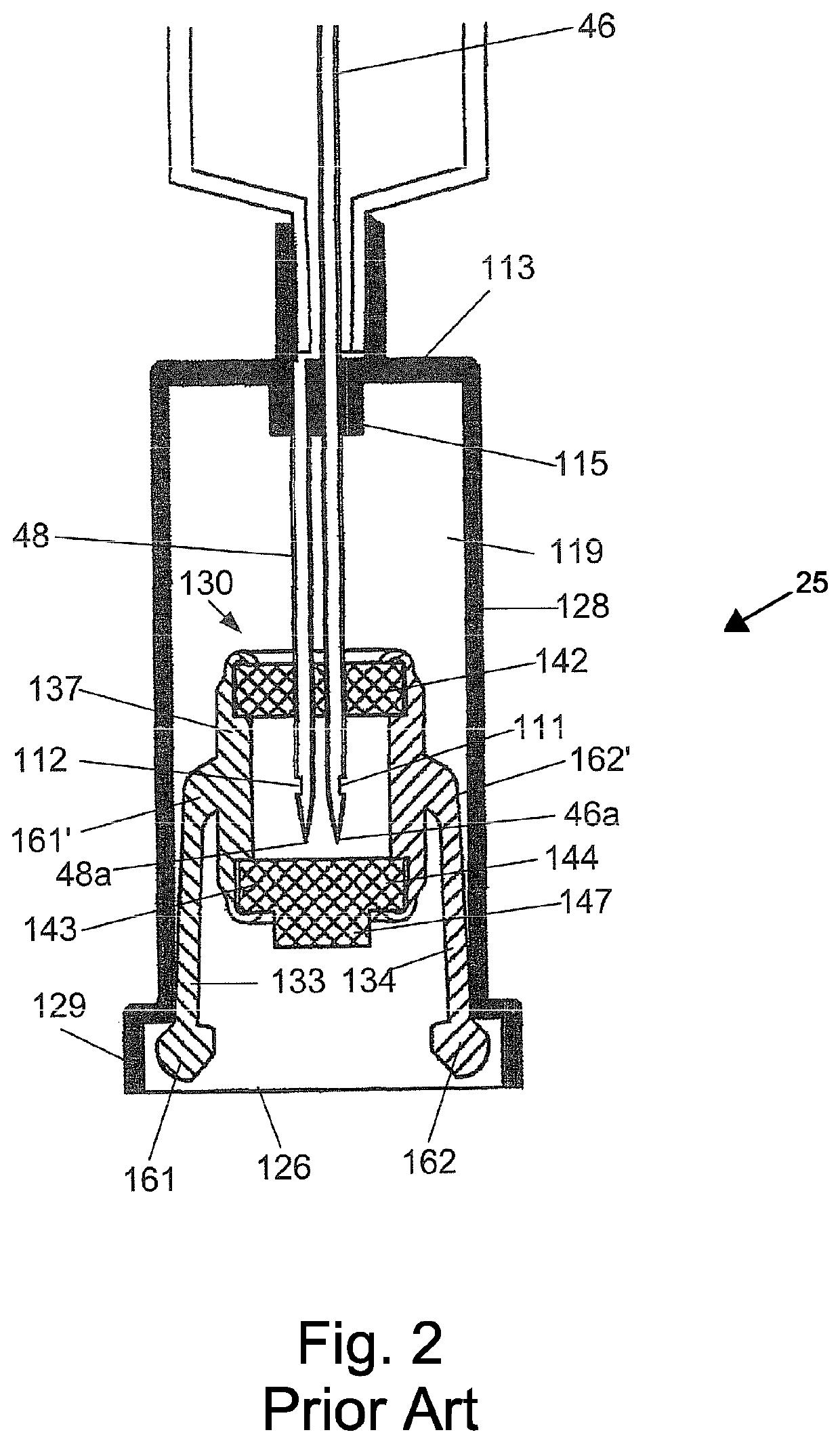
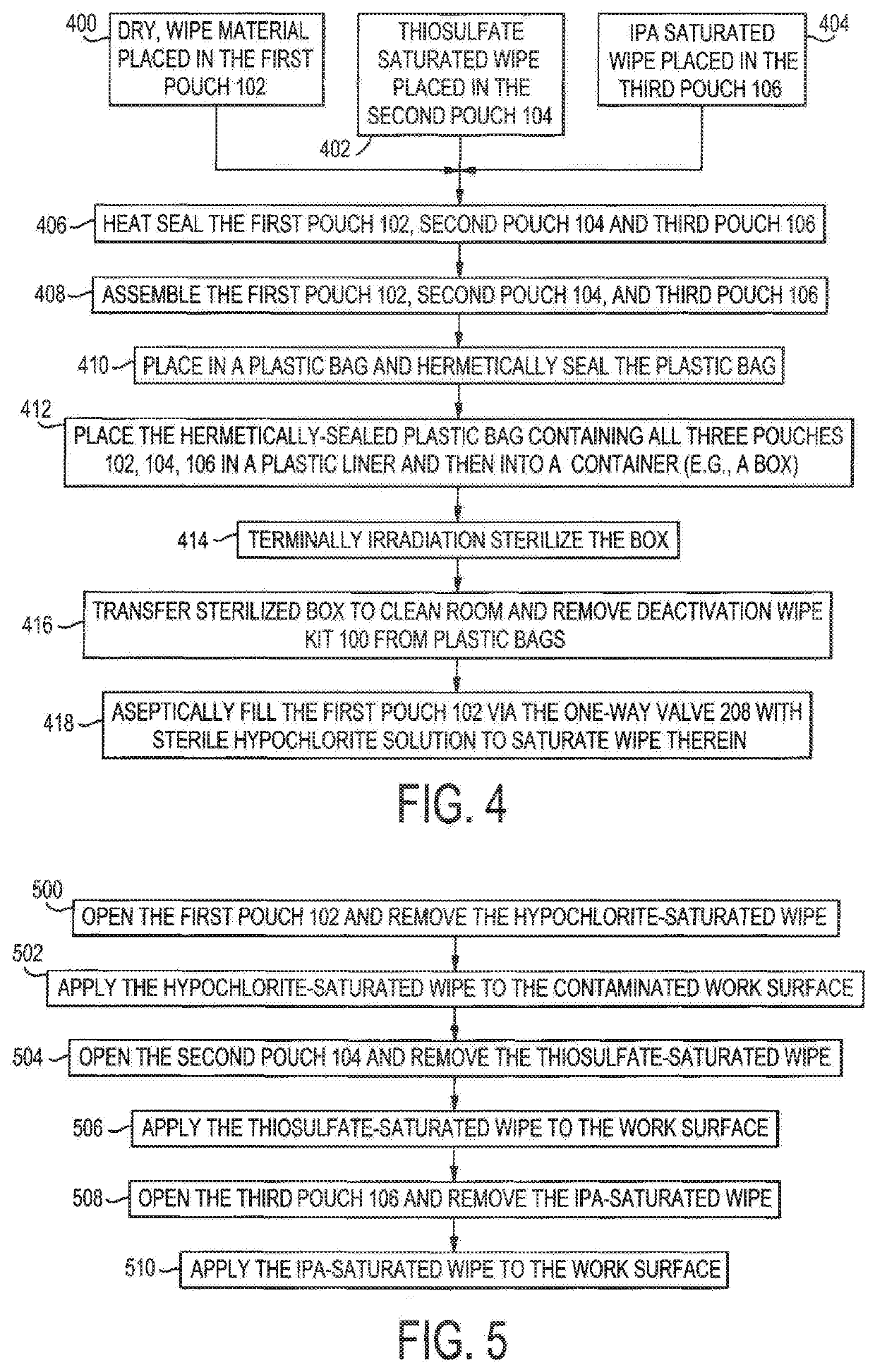
Deactivation wipe kit and method of forming and using the same
A hazardous drug deactivation wipe kit includes a first pouch having a one-way valve coupled to an end thereof, a second pouch, and a third pouch. The first pouch contains a wipe saturated in a hypochlorite solution, the second pouch contains a wipe saturated in thiosulfate solution, and the third pouch contains a wipe saturated in isopropyl alcohol solution. The deactivation wipe kit may be used in a clean room to deactivate most hazardous drugs on a work surface.
Owner:VALTEK ASSOC INC
Systems and methods for providing a closed venting hazardous drug IV set
A device for priming and venting a hazardous drug within an intravenous administration set (10). The device includes various access ports (42, 26, 86) and fluid channels (50, 150) to permit direct injection of a hazardous drug into the fluid reservoir (24), while eliminating the possibility of undesirable exposure to the hazardous drug. The device further includes priming and flushing ports to enable flushing of a hazardous drug from the system following an infusion procedure.
Owner:BECTON DICKINSON & CO
Deactivation wipe kit
A hazardous drug deactivation wipe kit includes a first pouch having a one-way valve coupled to an end thereof, a second pouch, and a third pouch. The first pouch contains a wipe saturated in a hypochlorite solution, the second pouch contains a wipe saturated in thiosulfate solution, and the third pouch contains a wipe saturated in isopropyl alcohol solution. The deactivation wipe kit may be used in a clean room to deactivate most hazardous drugs on a work surface.
Owner:VALTEK ASSOC INC
Venous transfusion drug dispensing center
PendingCN111519953AQuality assuranceImprove safety managementTelevision system detailsMechanical apparatusPN - Parenteral nutritionDrug dispensing
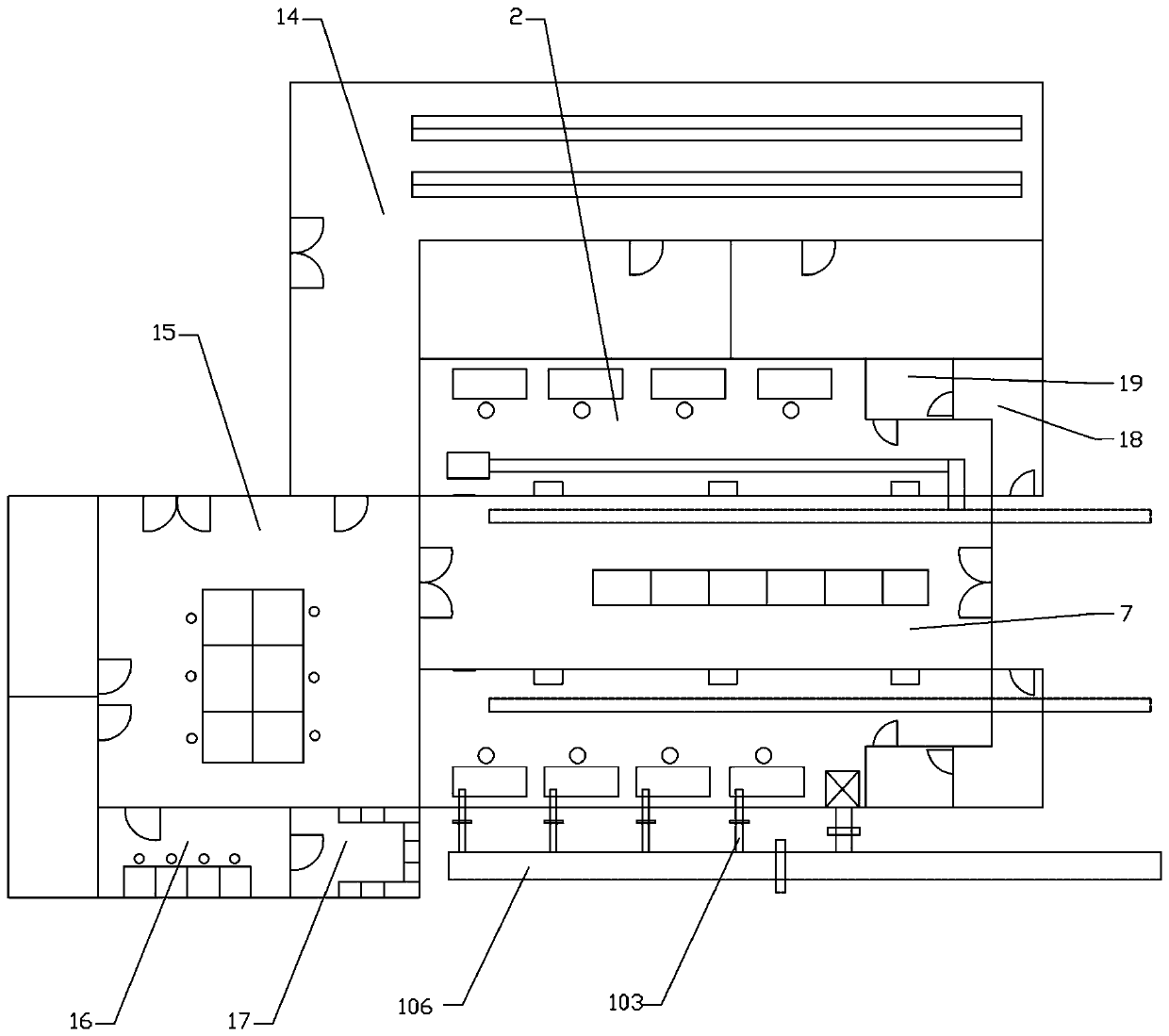
The invention relates to a venous transfusion drug dispensing center, and belongs to a medical facility in a hospital. According to the technical scheme, the venous transfusion drug dispensing centercomprises a cleaning area, an auxiliary working area and a living area; the cleaning area comprises a dispensing room which is separated from each other and sealed in space, and the dispensing room isdivided into a dispensing room of antibacterial and hazardous drugs and a dispensing room of ordinary drugs and parenteral nutrition; the auxiliary working area is further provided with a matched finished-product transfusion review area, a drug and material storage room, a verification party printing room, a dispensing preparation room, a finished-product verification room, a packaging room and ageneral dressing functional room, and isolation facilities need to be arranged in areas with different cleanliness levels to prevent cross contamination. According to the venous transfusion drug dispensing center, a biosafety operating platform required by negative pressure and conventional transfusion dispensing are uniformly arranged in the venous transfusion dispensing center, the quality of afinished transfusion is ensured, the occupational injury is reduced, the air conditioning division management of the conventional dispensing environment and negative pressure dispensing environment is adopted, and the leakage of a toxic and harmful chemical drug preparation is avoided.
Owner:李祥
Hazardous contaminant collection kit and rapid testing
ActiveCN111108214AComponent separationMicrobiological testing/measurementPharmaceutical drugEnvironmental engineering
Contamination detection systems, kits, and techniques are described for testing surfaces for the presence of hazardous contaminants, while minimizing user exposure to these contaminants. Even trace amounts of contaminants can be detected. A collection kit provides a swab that is simple to use, easy to hold and grip, allows the user to swab large areas of a surface, and keeps the user's hands awayfrom the surface being tested. The kit also provides open and closed fluid transfer mechanism to transfer the collected fluid to a detection device while minimizing user exposure to hazardous contaminants in the collected fluid. Contamination detection kits can rapidly collect and detect hazardous drugs, including trace amounts of antineoplastic agents, in healthcare settings at the site of contamination.
Owner:BECTON DICKINSON & CO
Hazardous contaminant collection kit and rapid testing
ActiveCN111108380ABioreactor/fermenter combinationsBiological substance pretreatmentsPharmaceutical drugEnvironmental engineering
Contamination detection systems, kits, and techniques are described for testing surfaces for the presence of hazardous contaminants, while minimizing user exposure to these contaminants. Even trace amounts of contaminants can be detected. A collection kit provides a swab that is simple to use, easy to hold and grip, allows the user to swab large areas of a surface, and keeps the user's hands awayfrom the surface being tested. The kit also provides open and closed fluid transfer mechanism to transfer the collected fluid to a detection device while minimizing user exposure to hazardous contaminants in the collected fluid. Contamination detection kits can rapidly collect and detect hazardous drugs, including trace amounts of antineoplastic agents, in healthcare settings at the site of contamination.
Owner:BECTON DICKINSON & CO
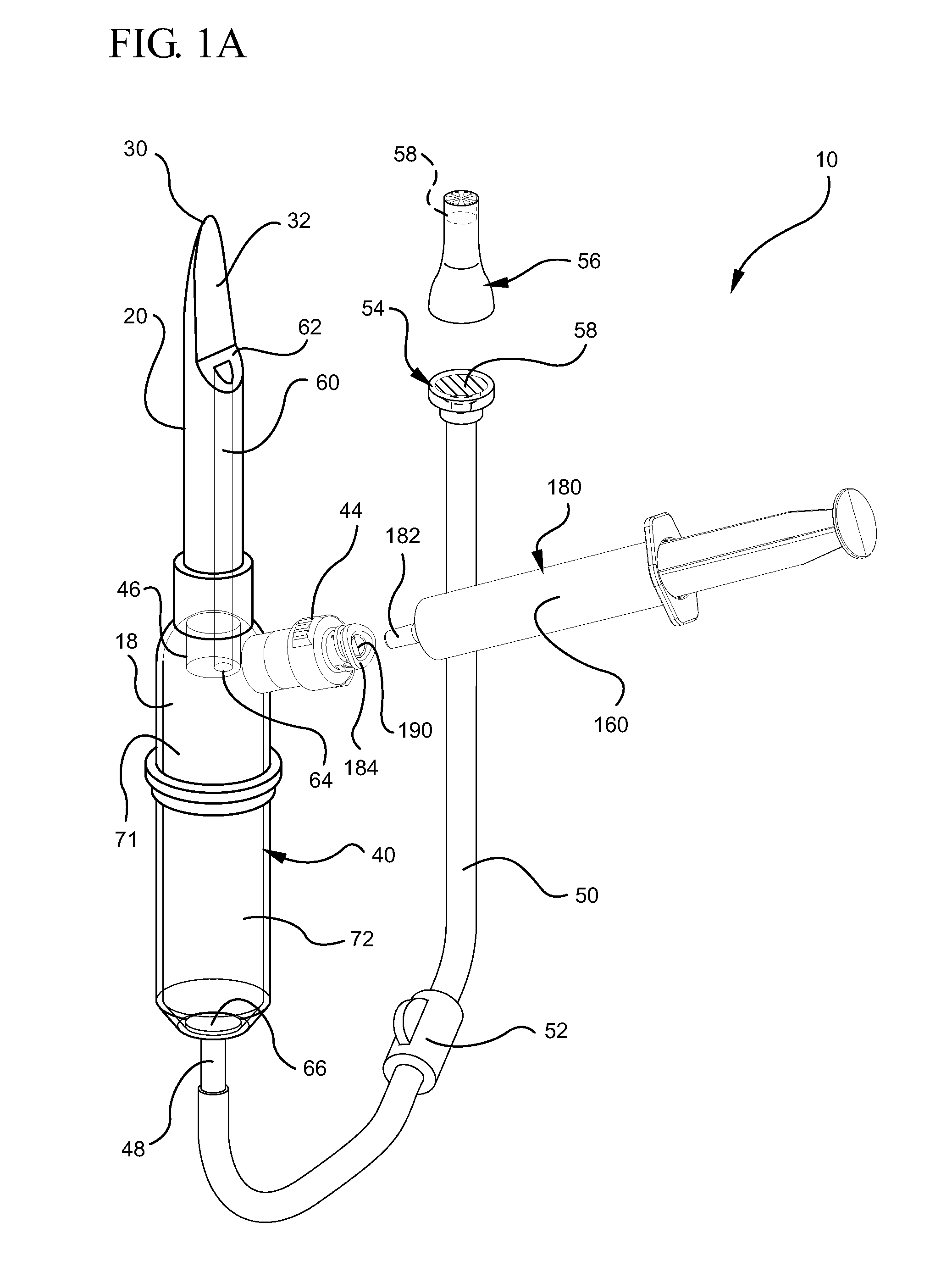
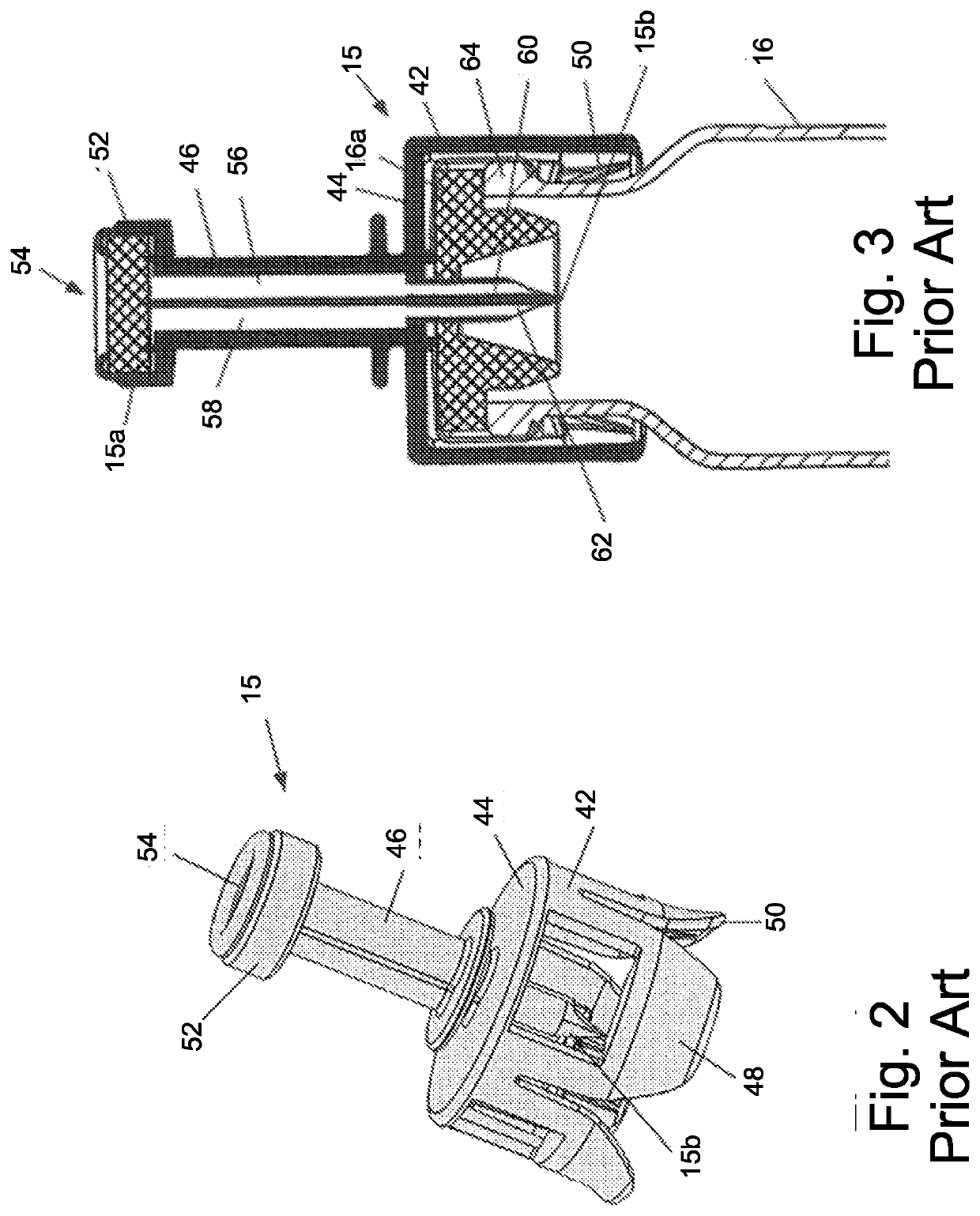
Connector section
ActiveUS11026864B2Obstruct passagePharmaceutical containersMedical packagingPharmaceutical drugEngineering
Owner:EQUASHIELD MEDICAL
Pouch with breakable seal
A wipe kit includes a pouch having a first compartment with a one-way valve coupled to an end thereof, a second compartment, and a frangible seal between the first and second compartments. The first compartment receives a liquid through the one-way valve and the second compartment contains a dry wipe. The frangible seal can be broken to permit liquid to flow from the first compartment onto the wipe in the second compartment. The deactivation wipe kit may be used in a clean room to deactivate most hazardous drugs on a work surface.
Owner:VALTEK ASSOC INC
Connector section
ActiveUS20190000718A1Obstruct passagePharmaceutical containersMedical packagingTransfer systemEngineering
A locking element for a connector configured to connect two components of a fluid transfer system and a connector that comprises the locking element are described. The connector comprising the locking element is configured to provide continuous fluid channels between a first component and a second component of a fluid transfer system, the fluid is a hazardous drug and the connector is designated for safe and contamination-free transfer of said hazardous drug from first to second container while isolating the needle tips and causing no dangerous and harmful leaks.
Owner:EQUASHIELD MEDICAL
Method and system for hazardous drug surface cleaning
ActiveUS11274271B2Cationic surface-active compoundsDetergent mixture composition preparationSurface cleaningPharmaceutical drug
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Method and system for hazardous drug surface cleaning
ActiveUS20200248111A1Cationic surface-active compoundsDetergent mixture composition preparationSurface cleaningPharmaceutical drug
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
System and method for hazardous drug surface cleaning
InactiveUS20150258578A1Non-surface-active detergent compositionsDetergent mixture composition preparationSurface cleaningHazardous drugs
The present invention relates to a method of cleaning a surface contaminated with a hazardous drug product involving cleaning in succession with a quaternary ammonium solution and an isopropyl alcohol solution.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Drug delivery device
This drug delivery device delivers a drug between a syringe barrel and a drug container, and, by enveloping the entire the drug container used in drug delivery, prevents exposure to hazardous drugs adhering to the drug container. This drug delivery device includes: a first member connected to the drug container on the side of a stopper; a second member connected to the first member and to the syringe barrel; and a third member connected to the first member and covering the drug container in a sealed state. The first member has a first hollow needle member and a drug container holder, and the second member has a second hollow needle member. The third member has an opening portion, a bottom surface portion where the drug container is stably mounted, and a flexible member which is connected to the opening portion and the bottom surface portion.
Owner:KOBAYASHI & CO LTD
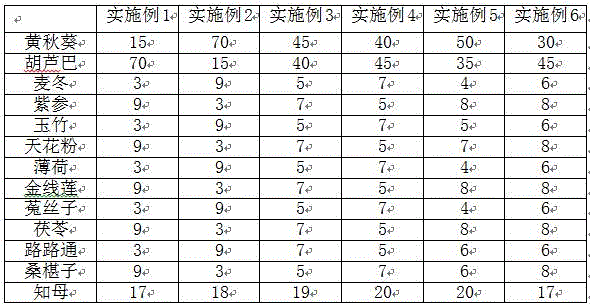
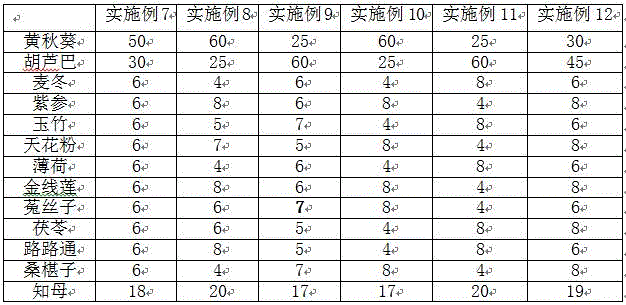
Chinese traditional medicine composition for preventing and treating pig constipation
InactiveCN106511769AReduce incidenceEasy to solveDigestive systemPlant ingredientsSide effectPeppermints
The invention discloses a Chinese traditional medicine composition for preventing and treating pig constipation. The Chinese traditional medicine composition is composed of, by weight, 10-70 parts of okra, 10-80 parts of fenugreek, 2-10 parts of radix ophiopogonis, 5-10 parts of salvia chinensis, 2-10 parts of radix polygonati officinalis, 2-10 parts of roots of Chinese trichosanthes, 2-10 parts of peppermint, 2-10 parts of roxburgh anoectochilus terminal buds, 2-10 parts of seeds of dodder, 2-10 parts of poria cocos, 2-10 parts of fructus liquidambaris, 2-10 parts of mulberry fruits and 17-20 parts of anemarrhenae. Material combination of the medicine is free of incompatibility, the occurrence rate of the pig constipation can be reduced obviously, good preventing and treating effects on the constipation are achieved, no toxic and side effect exists, using is safe, and no hazardous drug residue exists.
Owner:衡阳县轩健农牧发展有限公司
Drug pillow for treating cervical vertebra
The invention discloses a pillow, and particularly relates to a drug pillow for treating the cervical vertebra. According to the drug pillow for treating the cervical vertebra, potential hazardous drugs cannot be taken by a user, sleep time is fully utilized for achieving the aim of treatment, the treatment efficiency is high, and the treatment effect is good. The drug pillow comprises a head pillow part and a neck pillow part. The neck pillow part is columnar, one side of the head pillow part is connected with the column face of the neck pillow part in the axis direction, the diameter of the neck pillow part is larger than the thickness of the head pillow part, separation cloth is arranged in the neck pillow part in the diameter direction, and the interior of the neck pillow part is separated into a traditional Chinese medicine chamber and a filling chamber through the separation cloth. Neck protection traditional Chinese medicine is arranged in the traditional Chinese medicine chamber, and second filling chambers are arranged in the first filling chamber and the head pillow part, and filled with elastic filler.
Owner:肖鹏
Device for preventing accidental separation of a drip tube from an iv medication bag or bottle
InactiveUS20220023532A1Avoid accidental separationPrevent leakageInfusion devicesPharmaceutical containersWhole blood productHazardous drugs
Apparatus for preventing accidental separation of an IV tube or spike from an IV medication bag or bottle, thereby preventing spills or leakage of hazardous medication and / or biohazards. The apparatus includes a top clamp to attach around the stem of a medication bag or around the neck of a bottle, a bottom clamp to attach around a drip chamber of an IV tube, and a connecting portion connected to both the top clamp and the bottom clamp, the connecting portion ensuring that the top clamp cannot be pulled away from the bottom clamp. The top clamp can be removably attachable so as to enable clamping and unclam ping at the bag stem or bottle neck for situations requiring sequential bag changes, in the case of multiple blood products, or multiple IV bottles. Or, the top clamp can be permanently attachable, which is useful in the context of chemotherapy or immune therapy.
Owner:LANNAN TRACY A
Hazardous contaminant collection device with integrated swab and test device
PendingCN113383222APrevent proliferationAvoid exposureReagent containersMaterial analysis by observing effect on chemical indicatorPharmaceutical drugEnvironmental engineering
Contamination detection systems, kits, and techniques are described for testing surfaces for the presence of analytes of interest, including hazardous contaminants, while minimizing user exposure to these contaminants. Even trace amounts of contaminants can be detected. A collection device provides a swab that is simple to use, easy to hold and grip, allows the user to swab large areas of a surface, and keeps the user's hands away from the surface being tested. The collection device also includes a test strip, and provides a closed fluid transfer mechanism to transfer the collected fluid from the swab to the test strip while minimizing user exposure to hazardous contaminants in the collected fluid. Contamination detection kits can rapidly collect and detect hazardous drugs, including trace amounts of antineoplastic agents, in healthcare settings at the site of contamination.
Owner:BECTON DICKINSON & CO
Closed convenience kits for sterilized medicine preparation
PendingUS20220219842A1Safely and effectively provideDeteriorate fillingSurgical furniturePackage sterilisationPharmaceutical drugEngineering
Methods for providing a plurality of convenience kits for sterilizing and delivering a quantity of medicine into the safety of a sterile chamber inside closed system (which can be disposed in a potentially contaminating field environment) are disclosed for container filling applications including Avastin. fortified antibiotic eye drops, mitomycin and hazardous drugs, in general. The containers can include medical syringes, eye drop bottles, vials and product quality assurance containers. A syringe needle cap effective in containment of fluids dispensed from a syringe while being primed is also disclosed.
Owner:THORNE INTPROP HLDG LLC
Traditional Chinese medicine composition for preventing and treating ascites disease of pig frogs and preparation method of traditional Chinese medicine composition
InactiveCN106474311AReduce incidenceEasy to solveAnthropod material medical ingredientsFungi medical ingredientsDiseaseSide effect
Traditional Chinese medicine composition for preventing and treating ascites disease of pig frogs is prepared from components in parts by weight as follows: 20-40 parts of folium mori, mushroom and ganoderma mixed paste, 5-10 parts of silkworm chrysalis meal, 5-10 parts of persimmon, 5-10 parts of red radish, 5-10 parts of fructus crataegi, 5-10 parts of fresh dandelion, 5-10 parts of Noni, 5-10 parts of bitter gourd and 5-10 parts of rhizoma dioscoreae. Compatibility of the raw materials in the composition is guaranteed, the incidence rate of the disease of the pig frogs can be reduced obviously, and the composition has very good preventing and treating effects on the ascites disease of the pig frogs, has no toxic or side effects, is safe to use and is free of hazardous drug residues.
Owner:衡阳茂晨农业开发有限公司
Components of open liquid drug transfer systems and a robotic system employing them
PendingUS20220257470A1Improve securityProgramme-controlled manipulatorPharmaceutical containersDrug ContaminationRobotic arm
Presented herein are a robotic system that is configured for compounding and preparation of medications comprising non-hazardous drugs and a vented drug vial adapter. The robotic system comprises: a laminar flow cabinet; and at least one robotic arm. The vented drug vial adapter is designed to connect a drug vial to another component of a drug transfer system. The adapter comprises a hydrophobic filter that prevents passage of liquid while allowing air to pass through it and a vent hole to the atmosphere. The vent hole is located above the filter thereby allowing equalization of the internal pressure while preventing the drug from contaminating the atmosphere.
Owner:EQUASHIELD MEDICAL
System and method for hazardous drug surface cleaning
InactiveUS20160145549A1Detergent mixture composition preparationCleaning using toolsSurface cleaningHazardous drugs
The present invention relates to a method of cleaning a surface contaminated with a hazardous drug product involving cleaning in succession with a quaternary ammonium solution and an isopropyl alcohol solution.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com