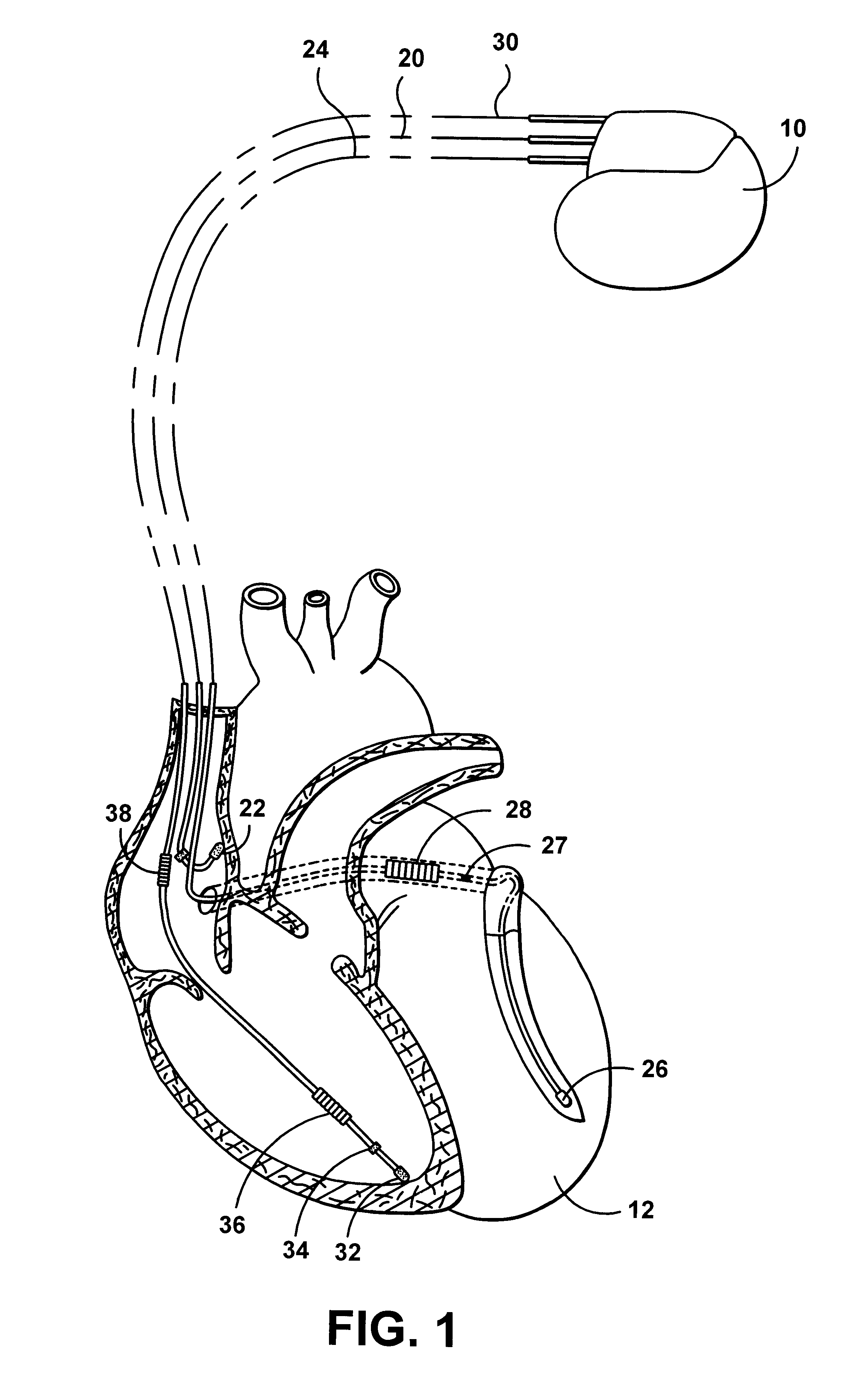
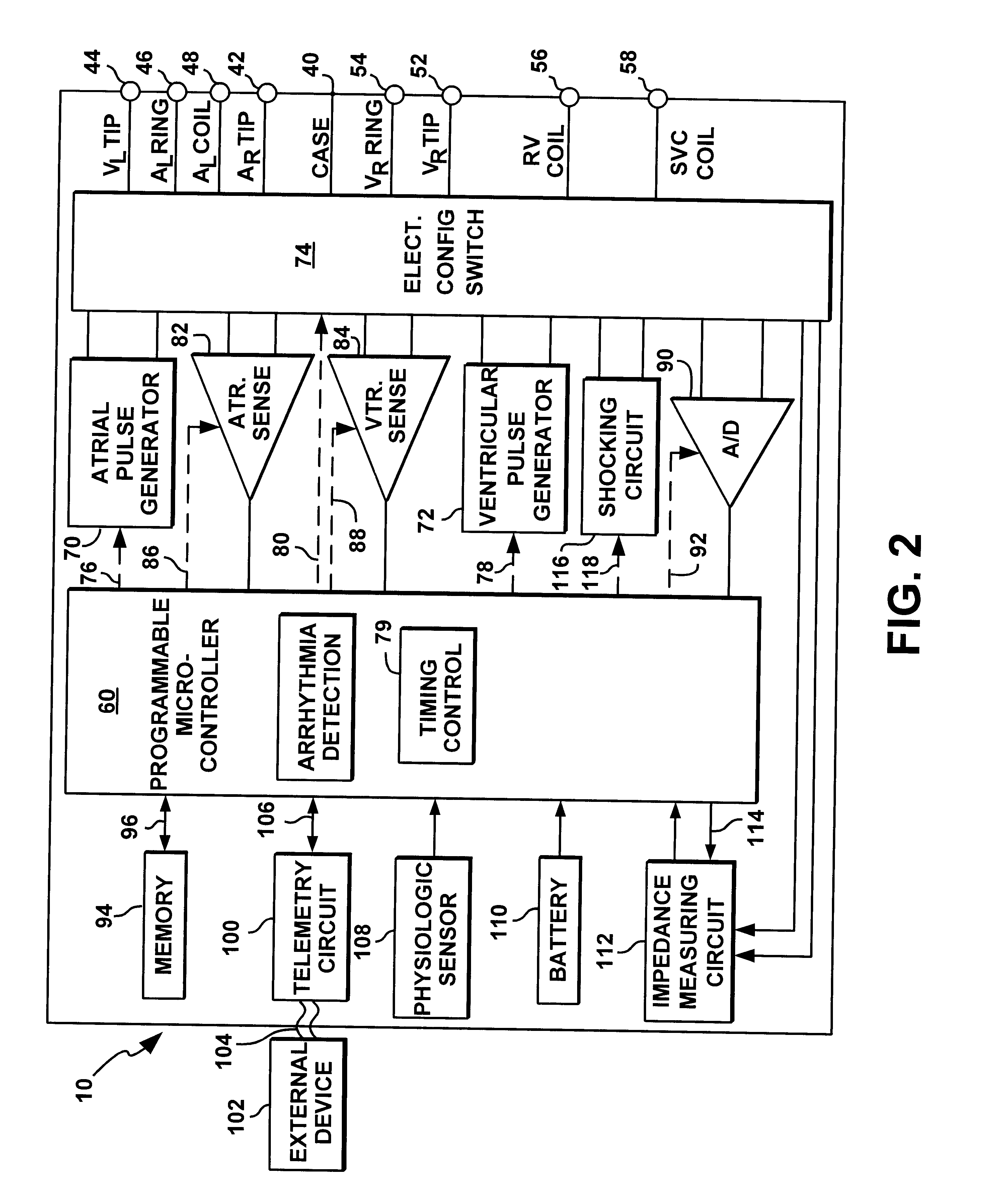
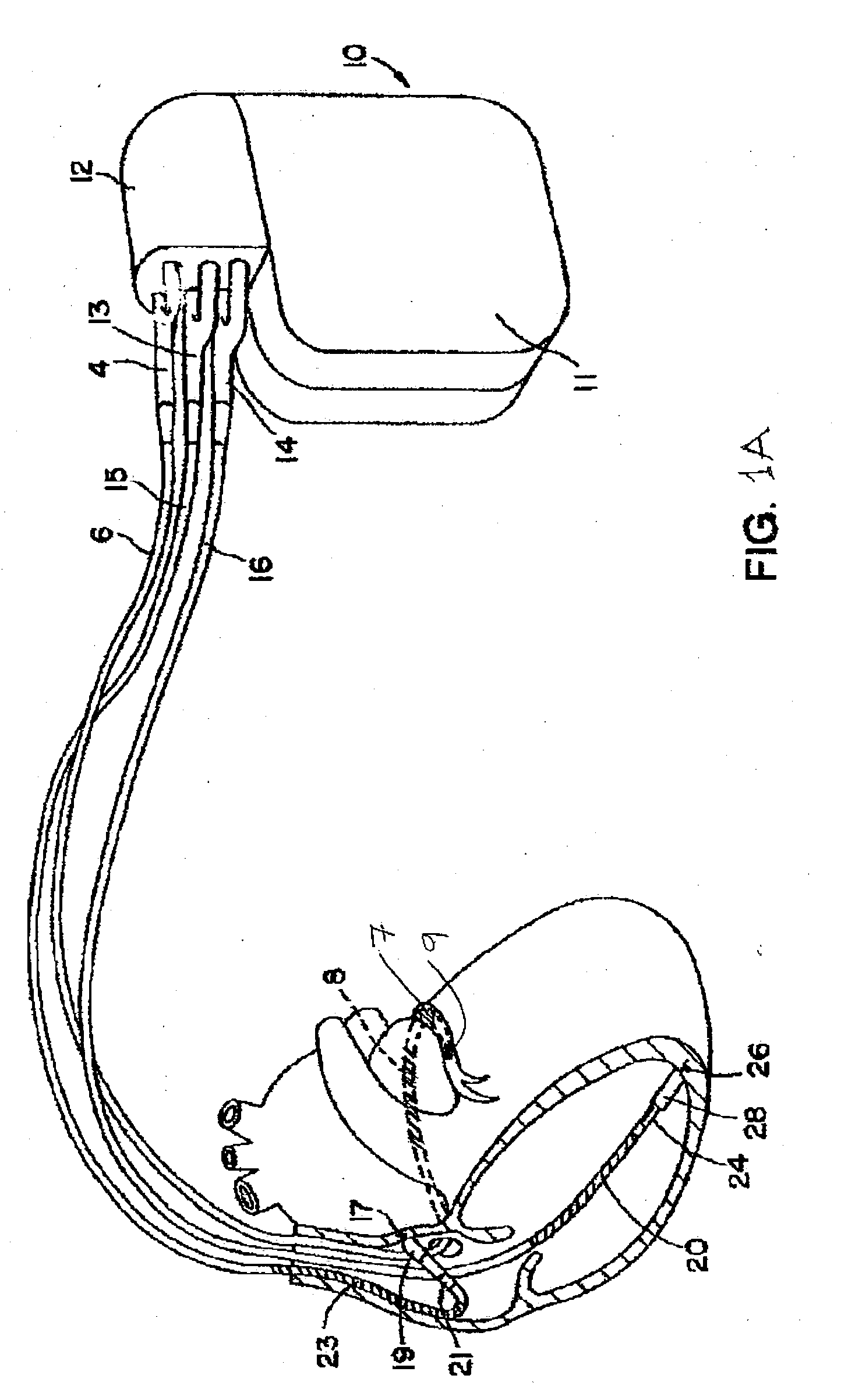
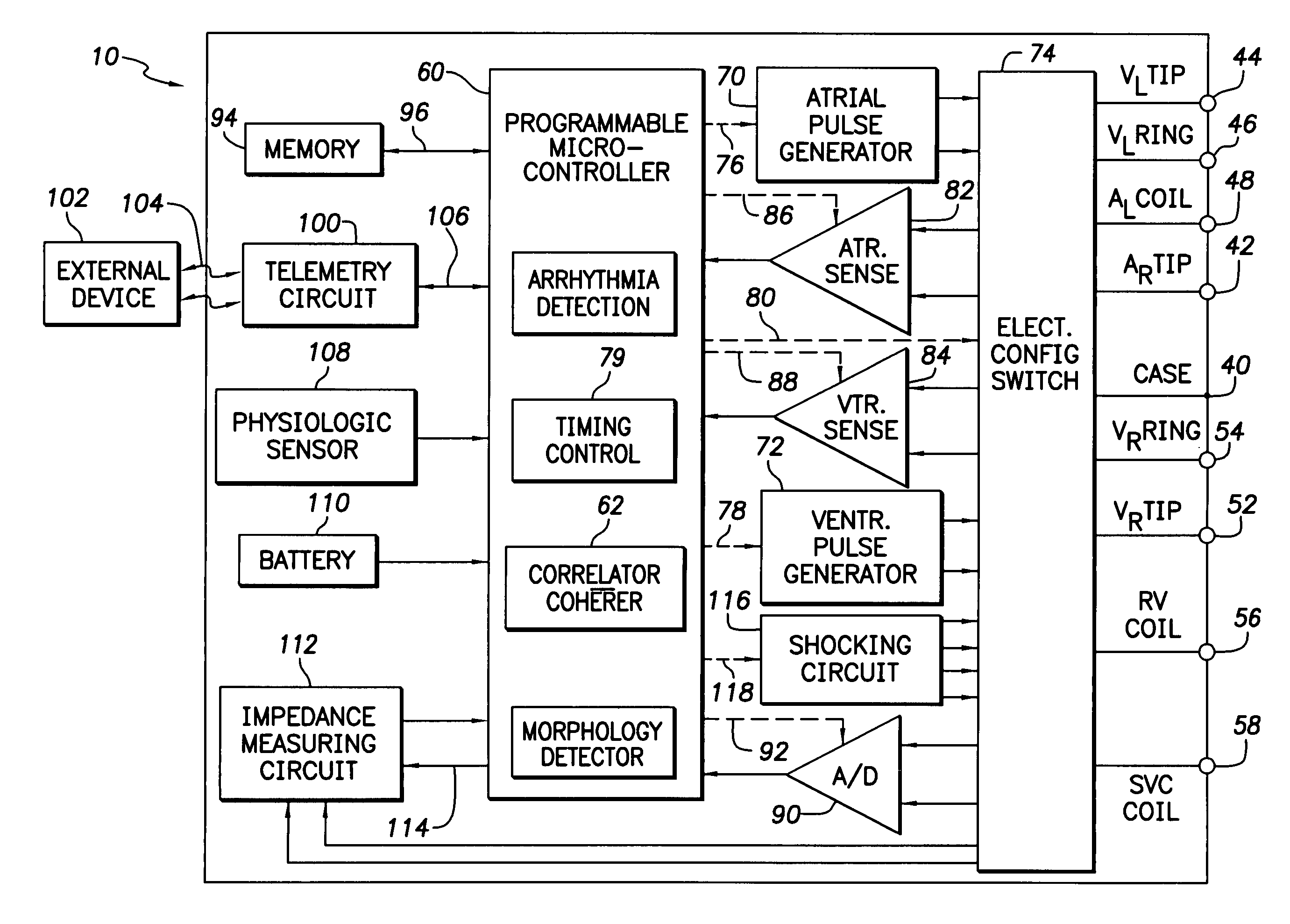
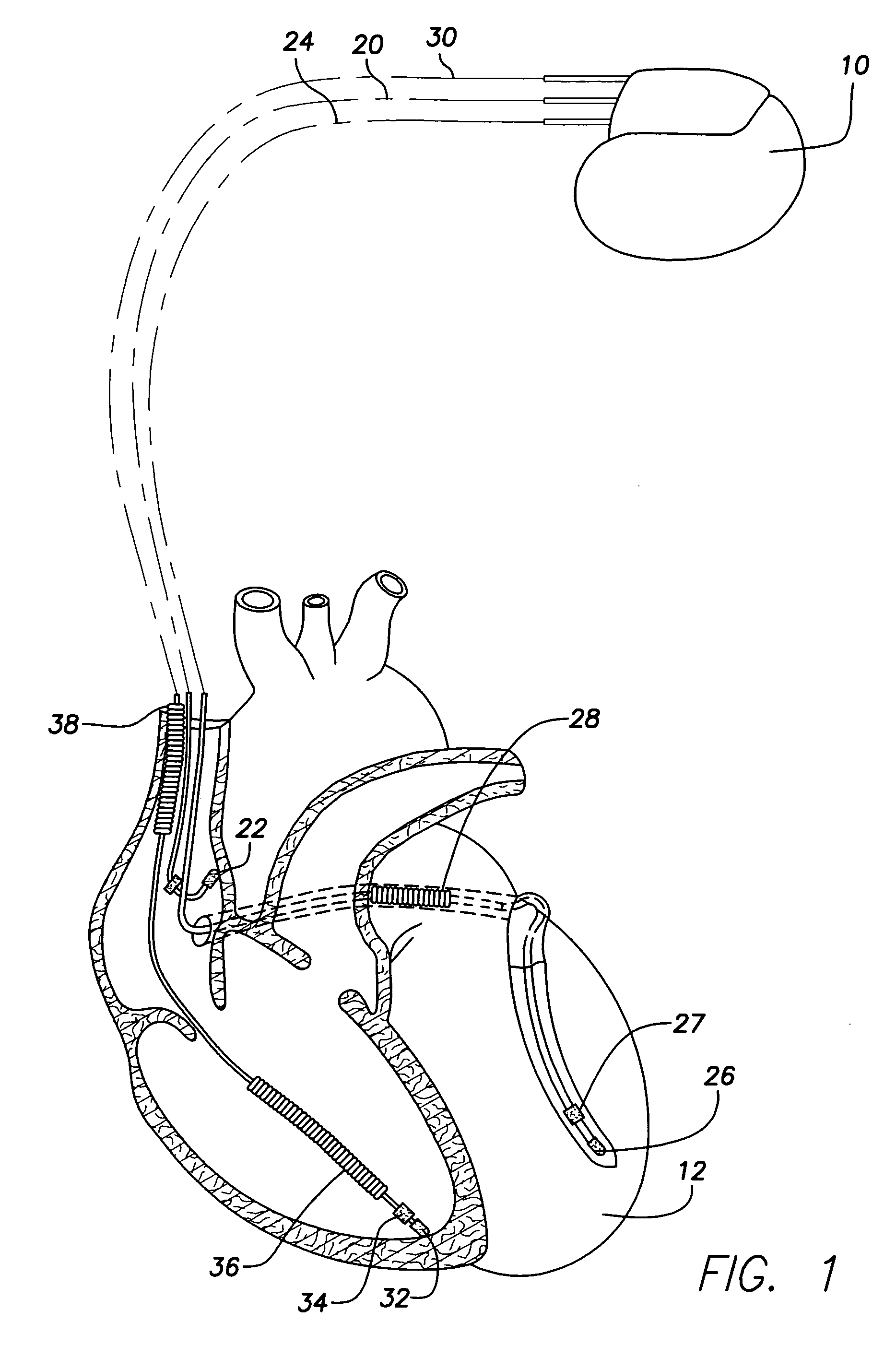
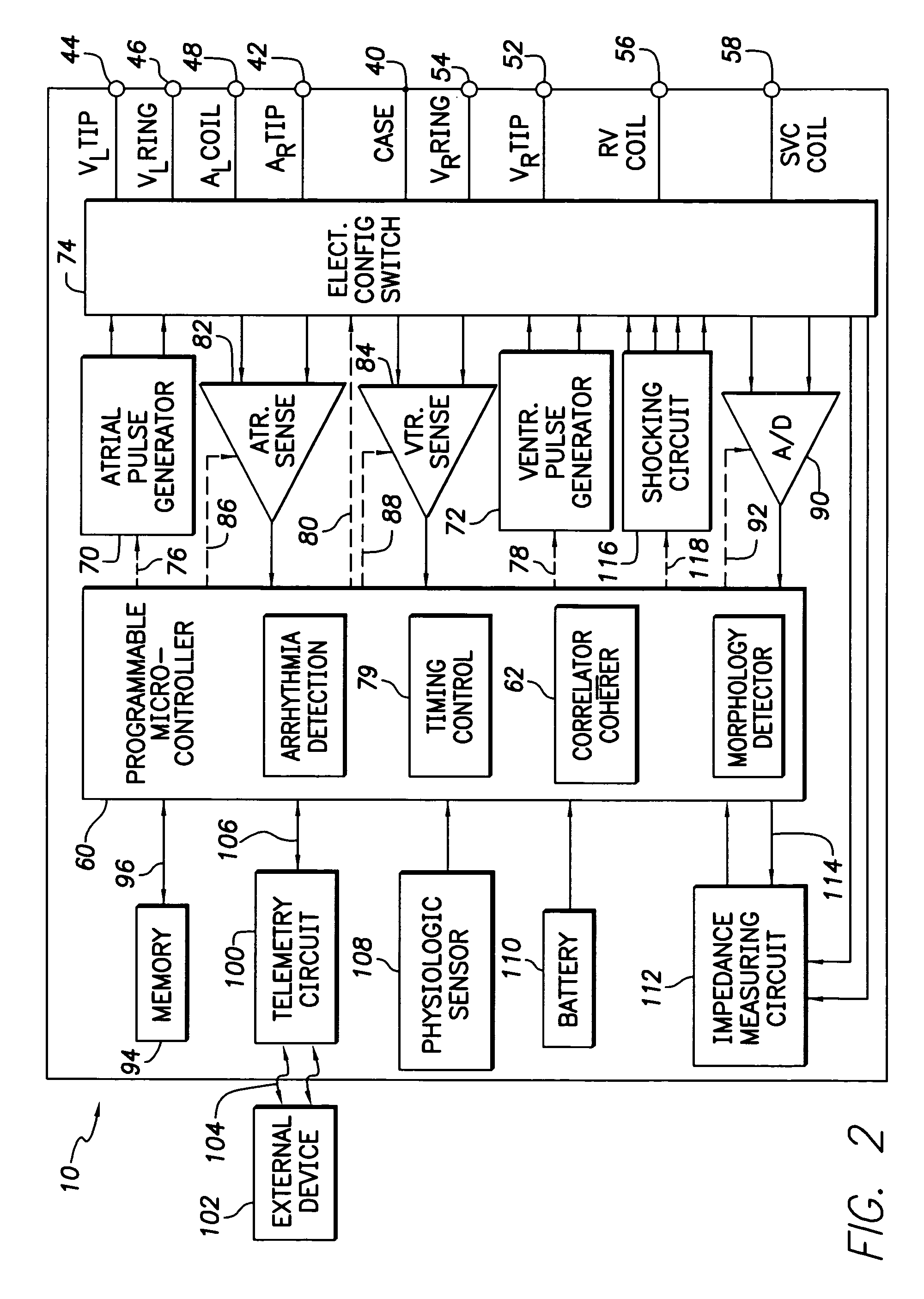
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
380 results about "Cardiac stimulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
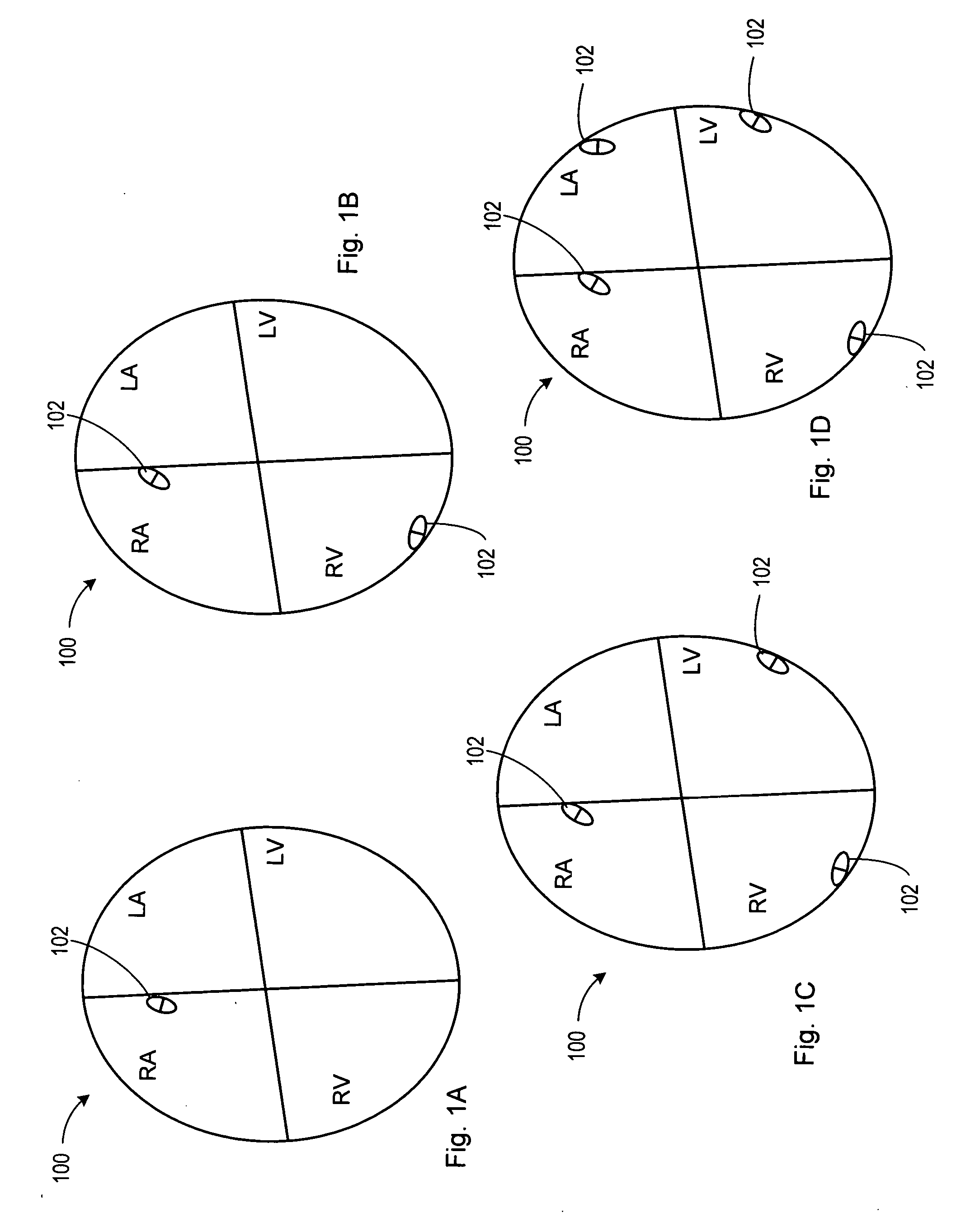
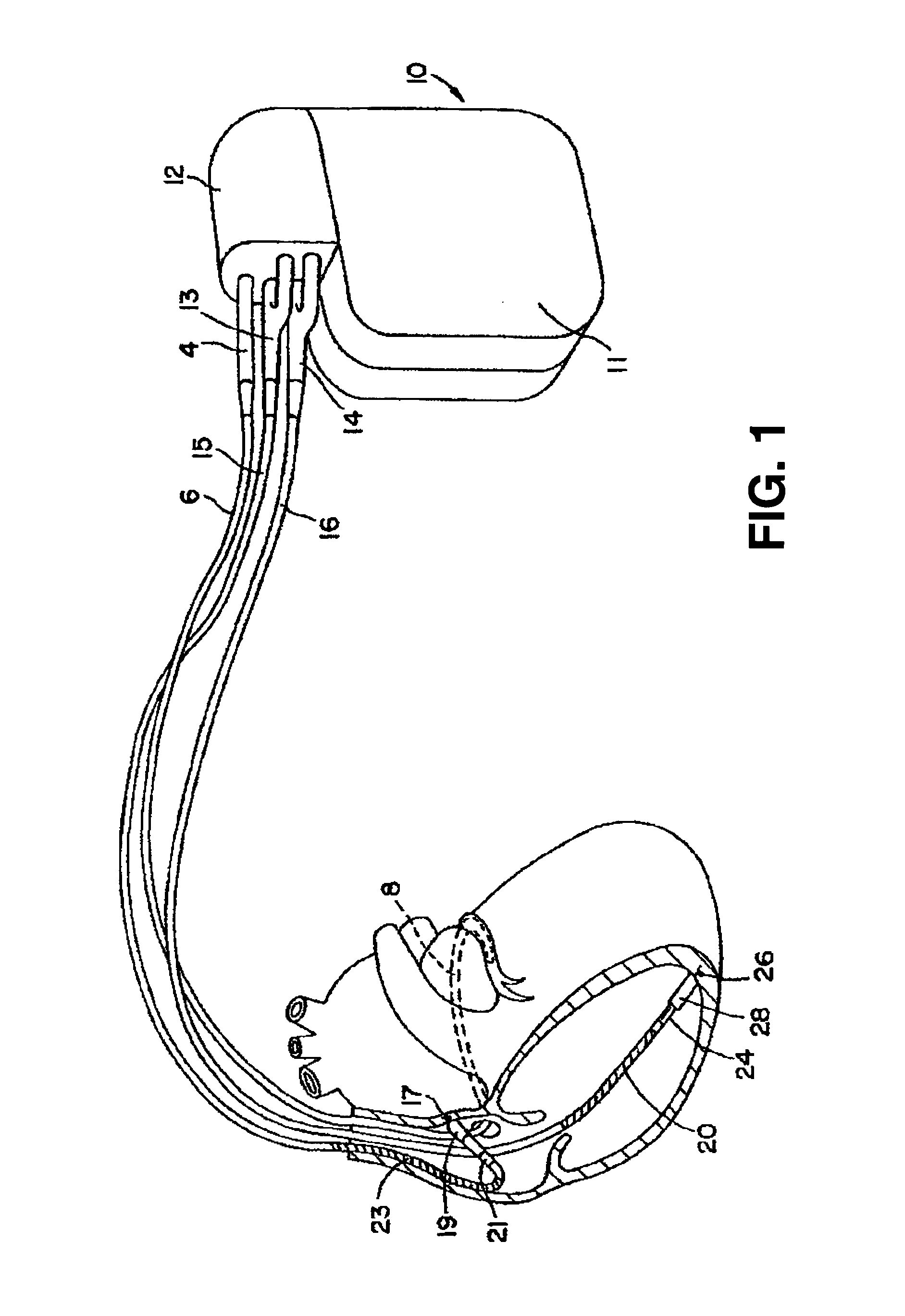
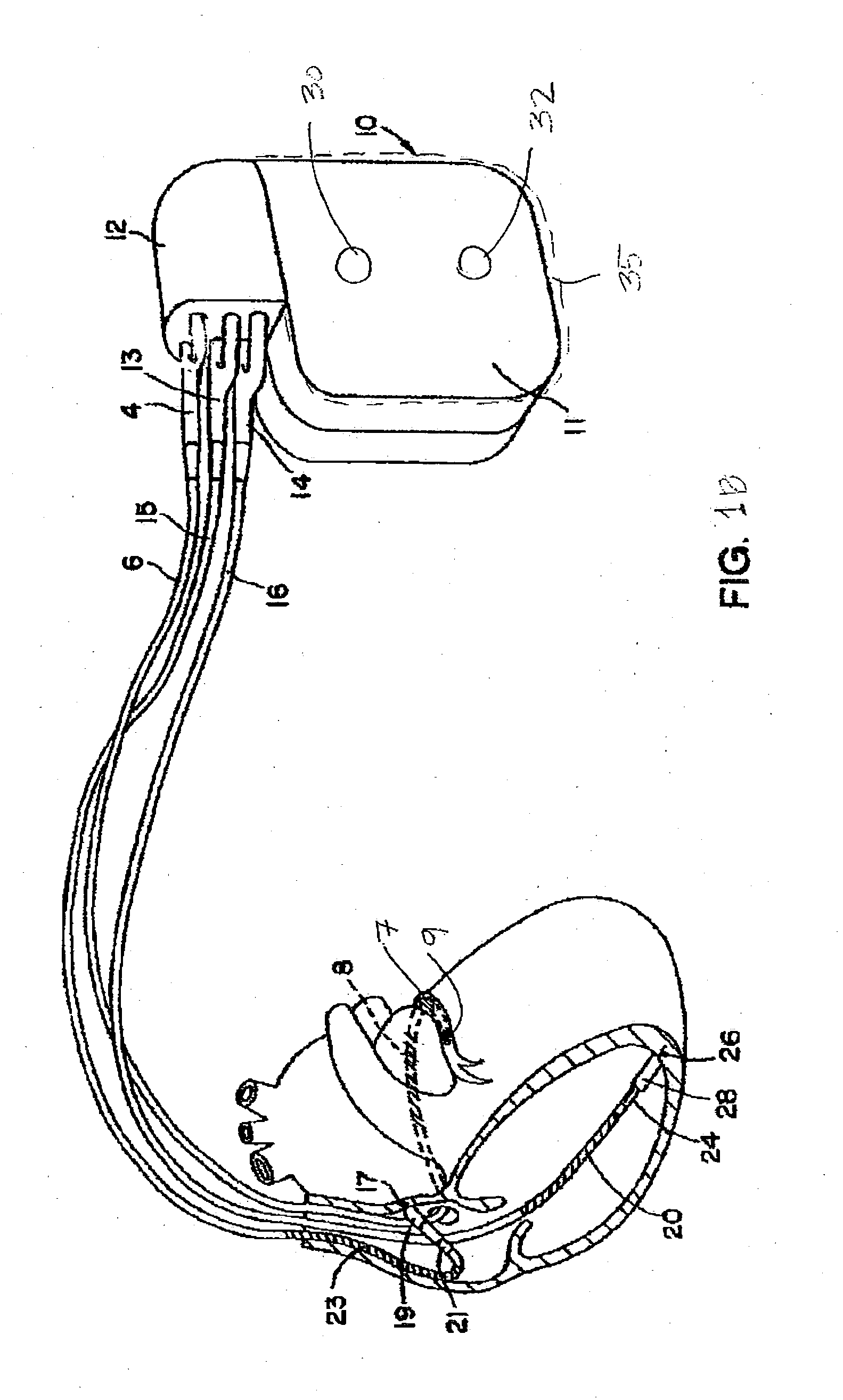
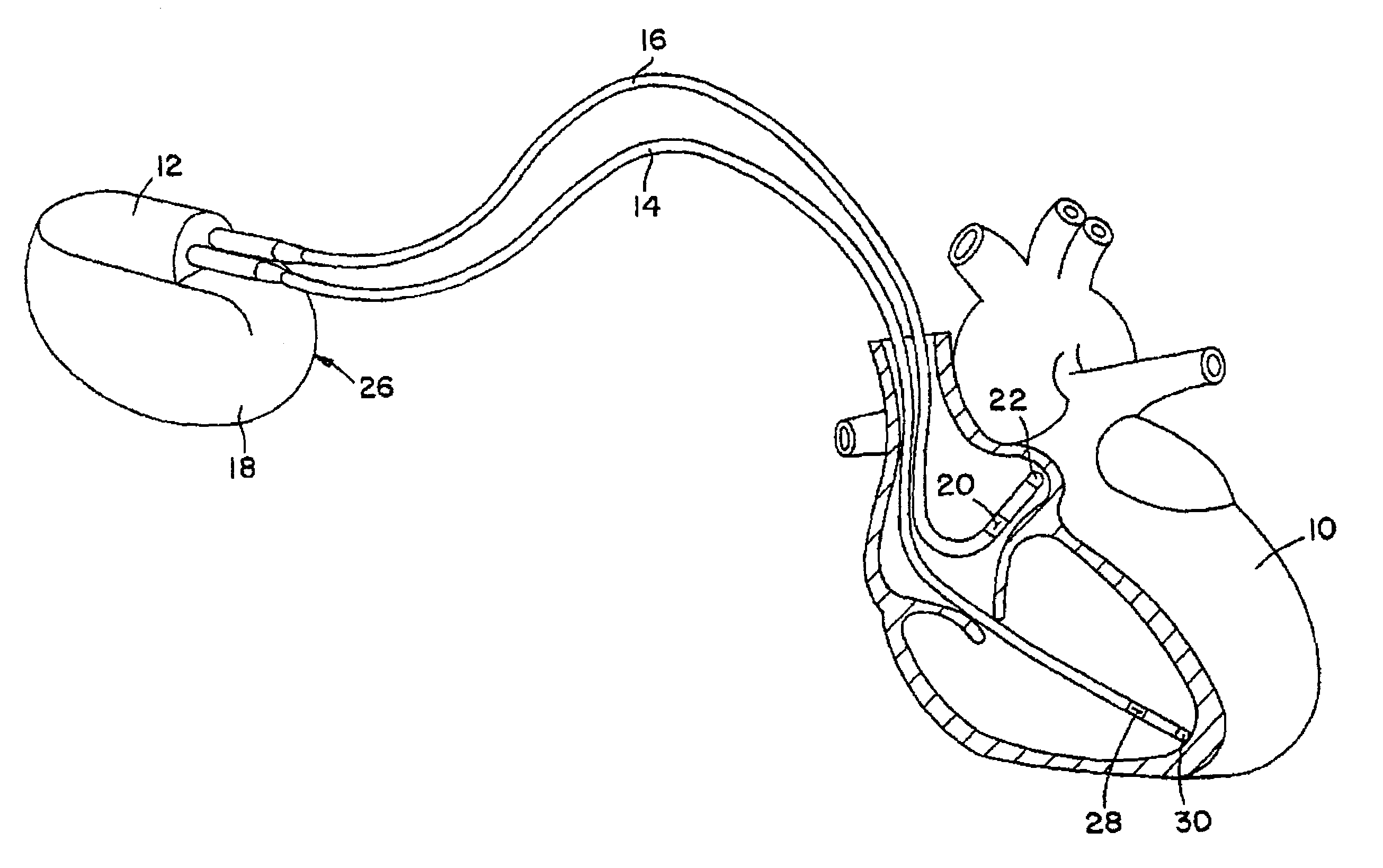
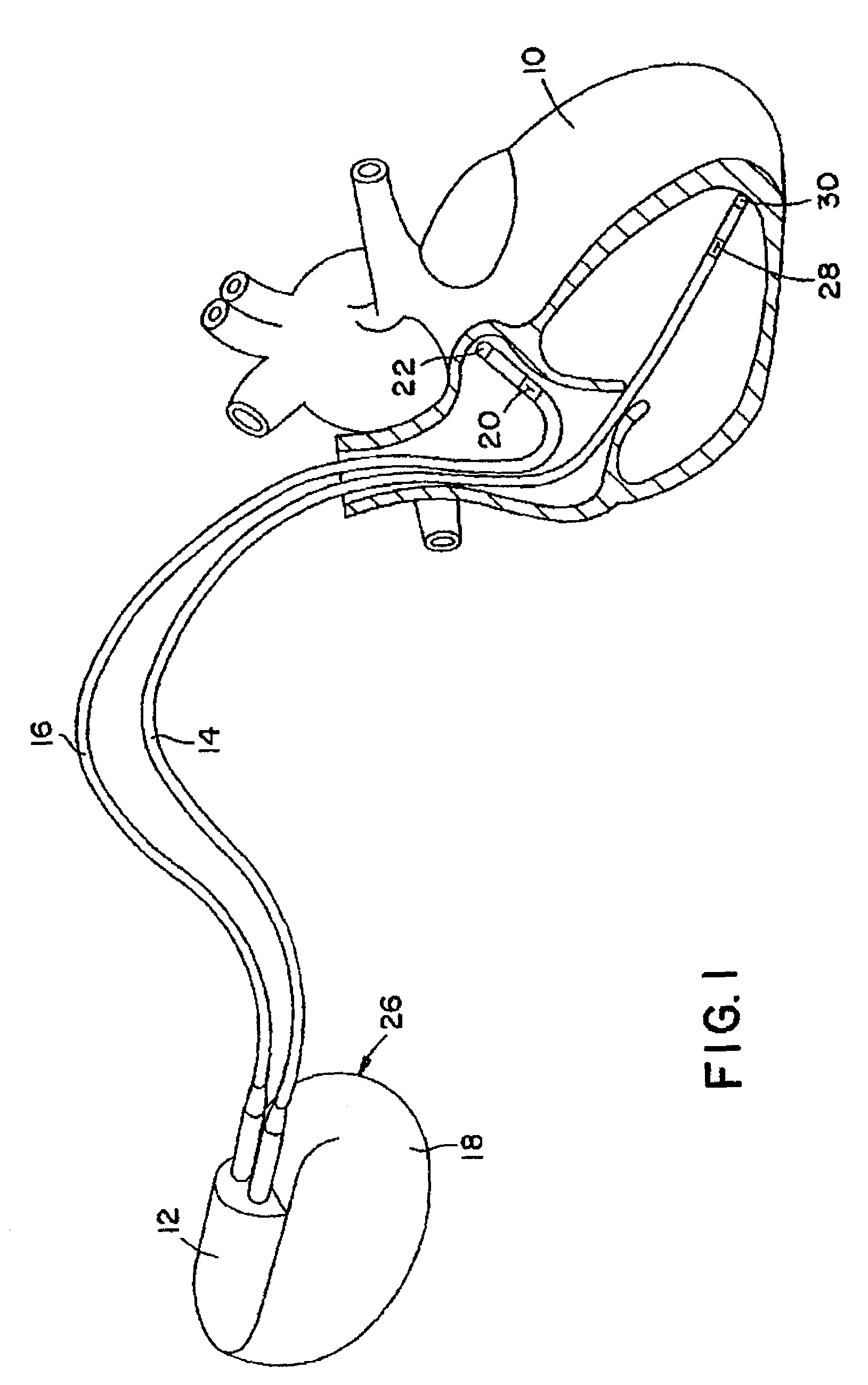
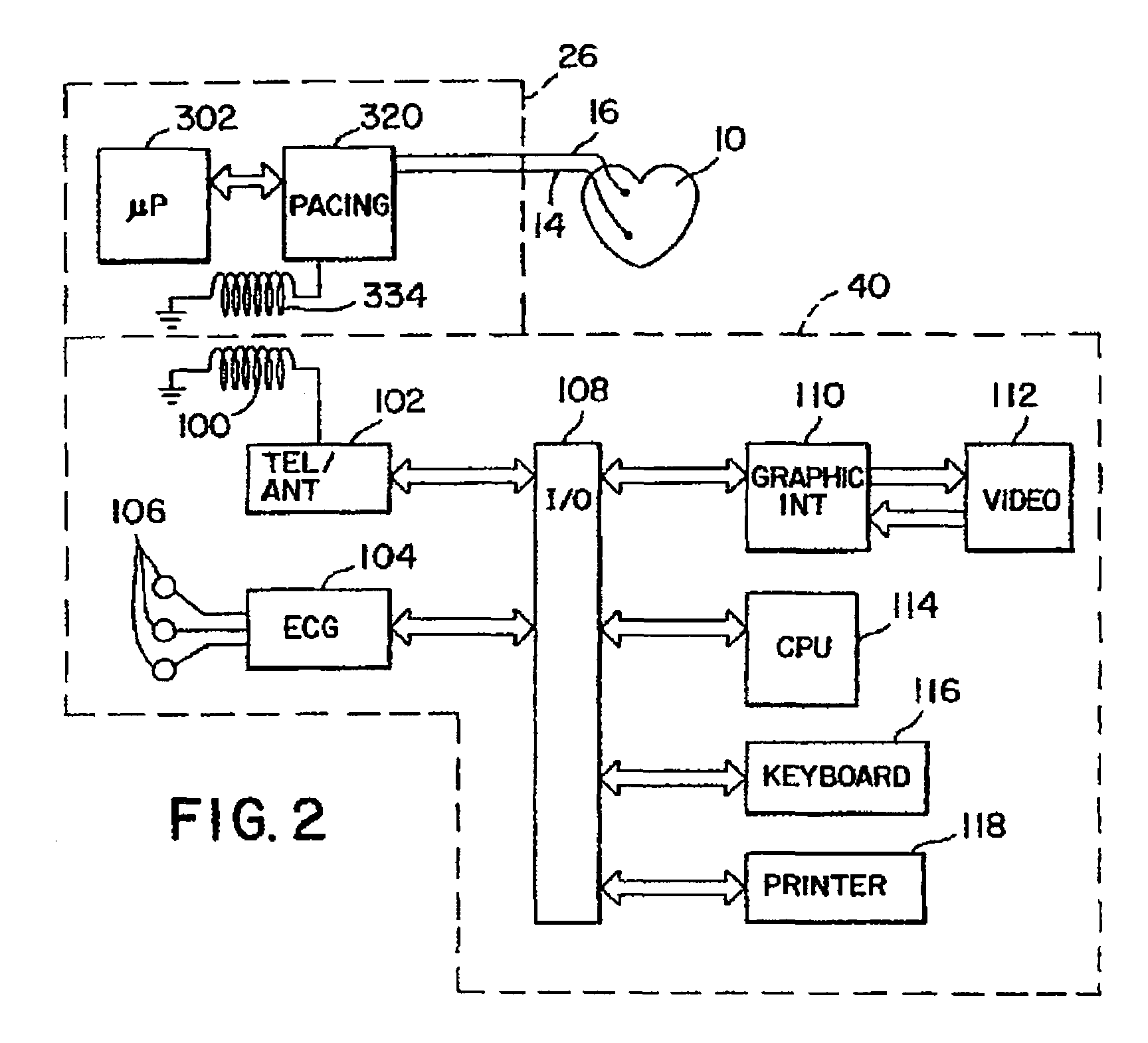
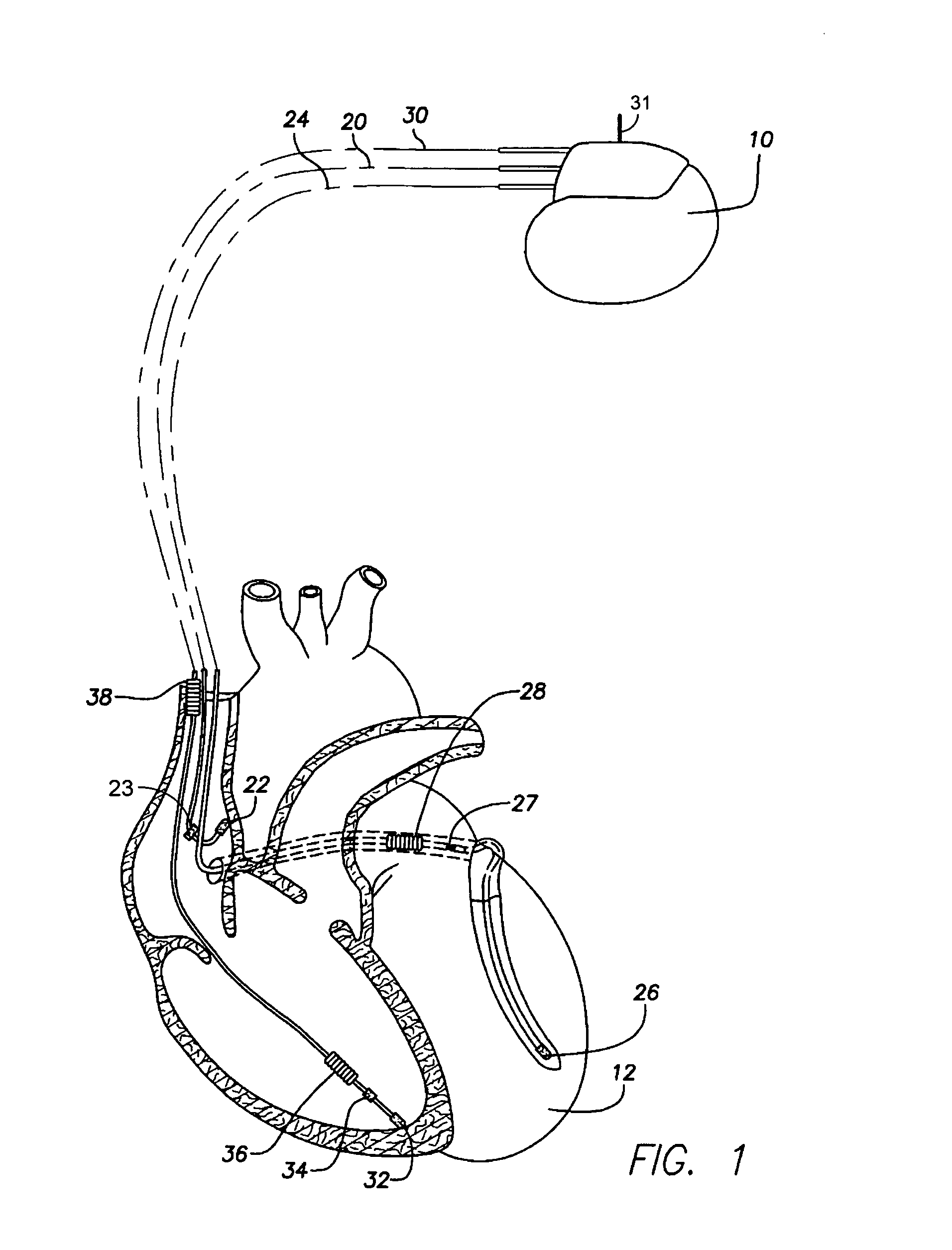
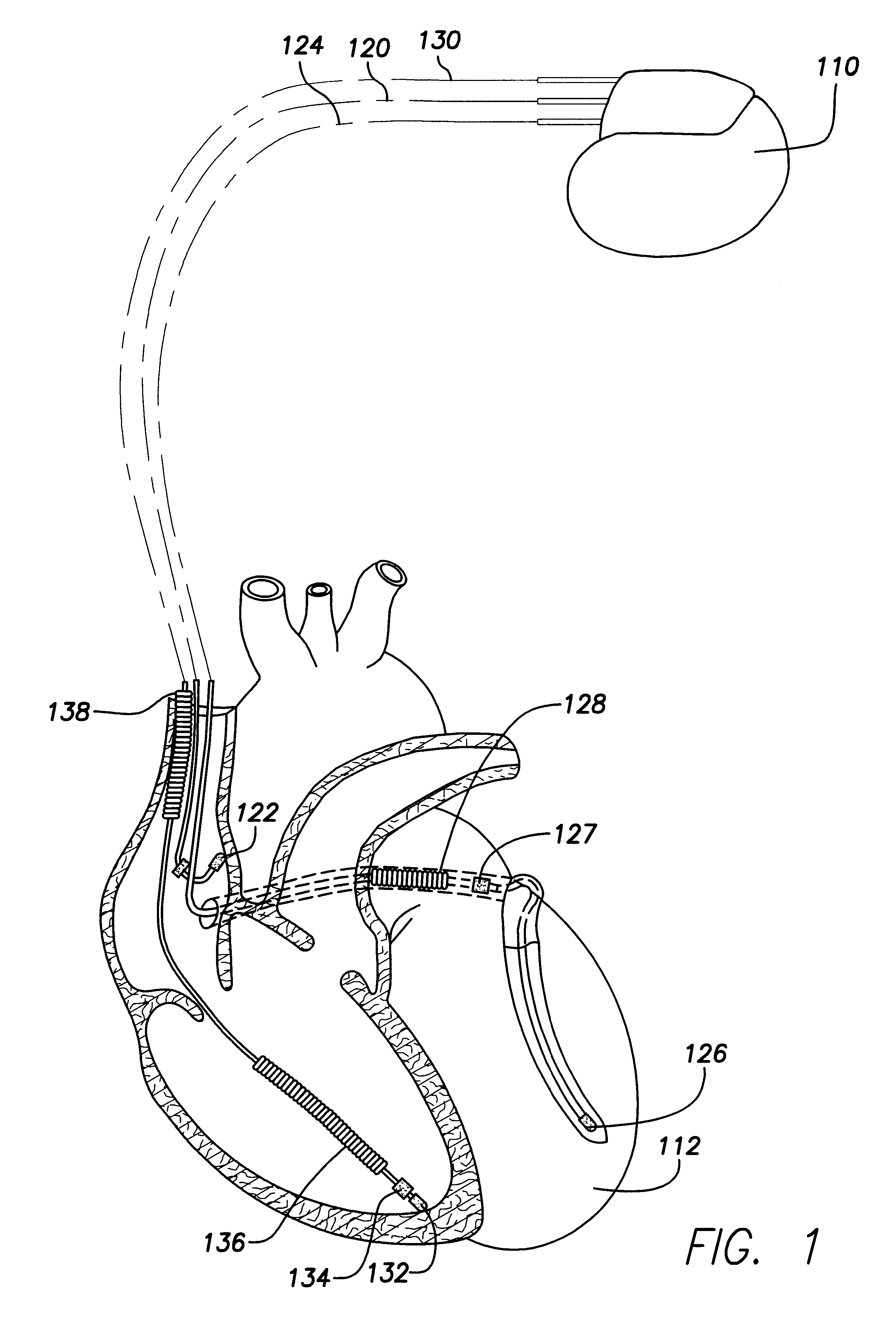
Stimulators Cardiac stimulation is carried out by delivering a pulse of electrical current through the electrode catheter from an external pacemaker (stimulator) to the cardiac surface. Such an electrical impulse depolarizes cardiac tissue near the pacing electrode, which then propagates through the heart.
Stimulation device for sleep apnea prevention, detection and treatment
InactiveUS6928324B2Increase oxygen concentrationReduce carbon dioxide concentrationHeart stimulatorsArtificial respirationSleep apneaMetabolic demand
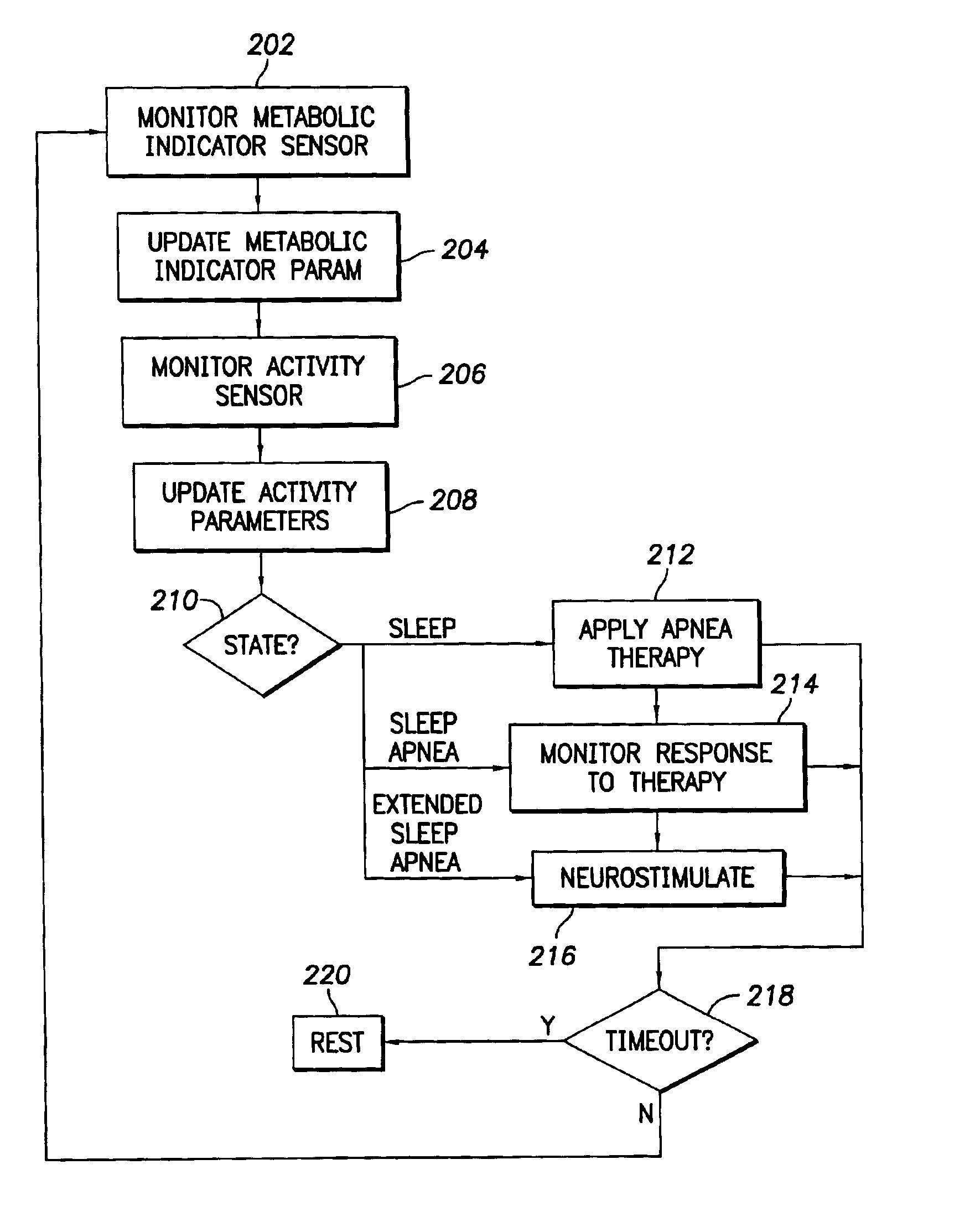
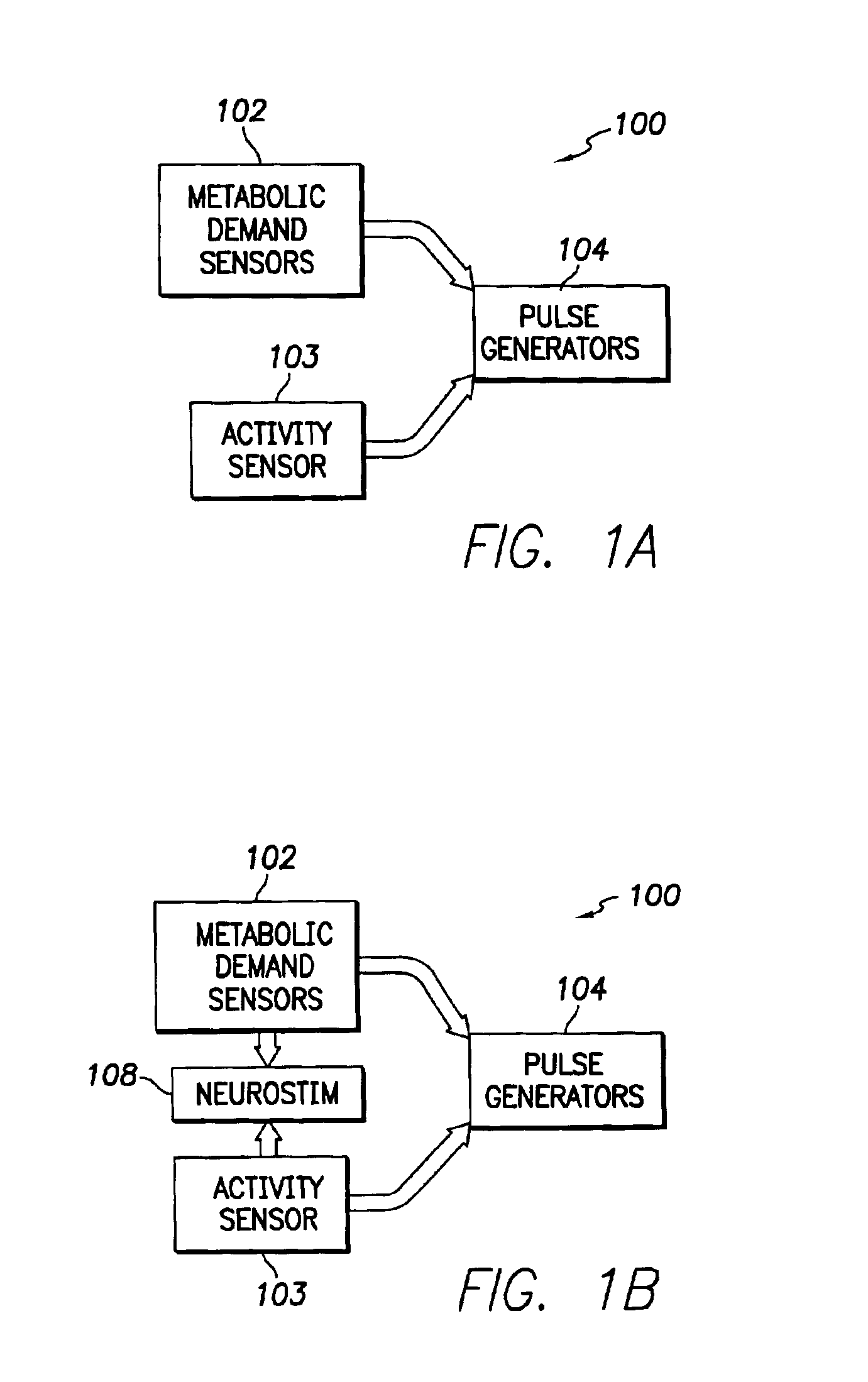
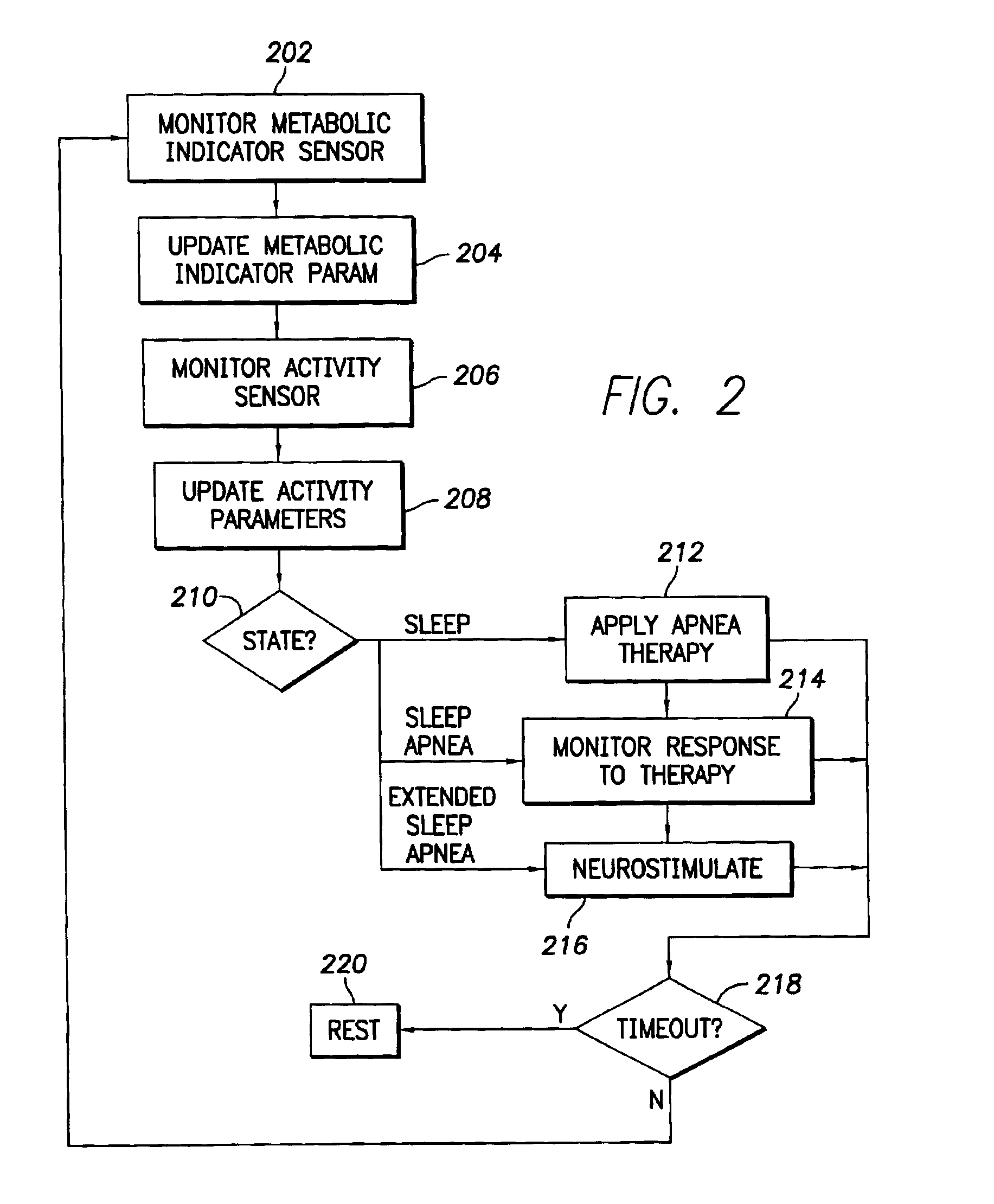
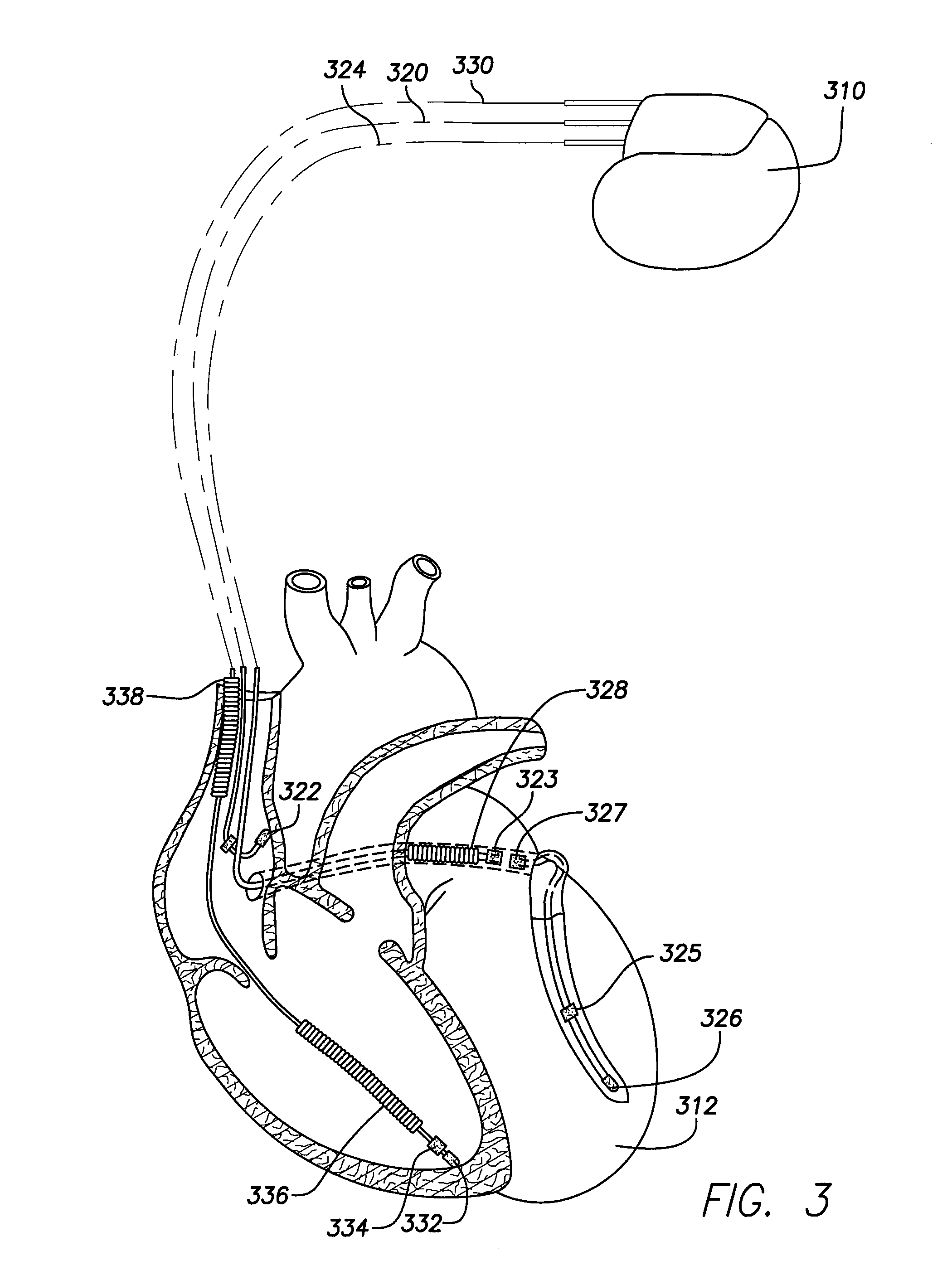
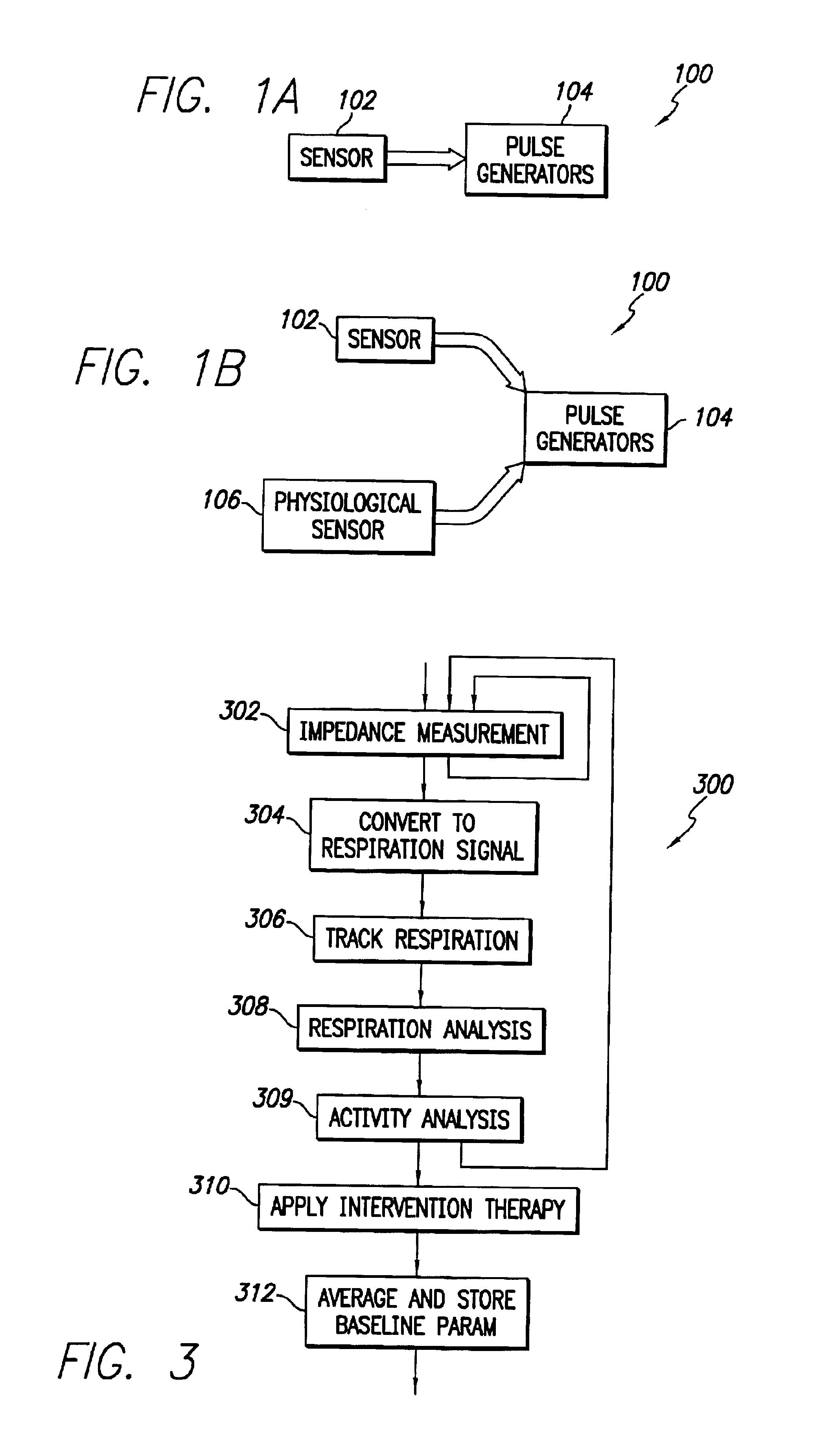
An implantable cardiac stimulation device comprises a metabolic demand sensor, an activity sensor, and one or more pulse generators. The metabolic demand sensor and activity sensor can sense metabolic demand and physical activity parameters, respectively. The pulse generators can generate cardiac pacing pulses with timing based on a comparison of the metabolic demand and physical activity parameters. The timed cardiac pacing pulses can prevent a sleep apnea condition.
Owner:PACESETTER INC
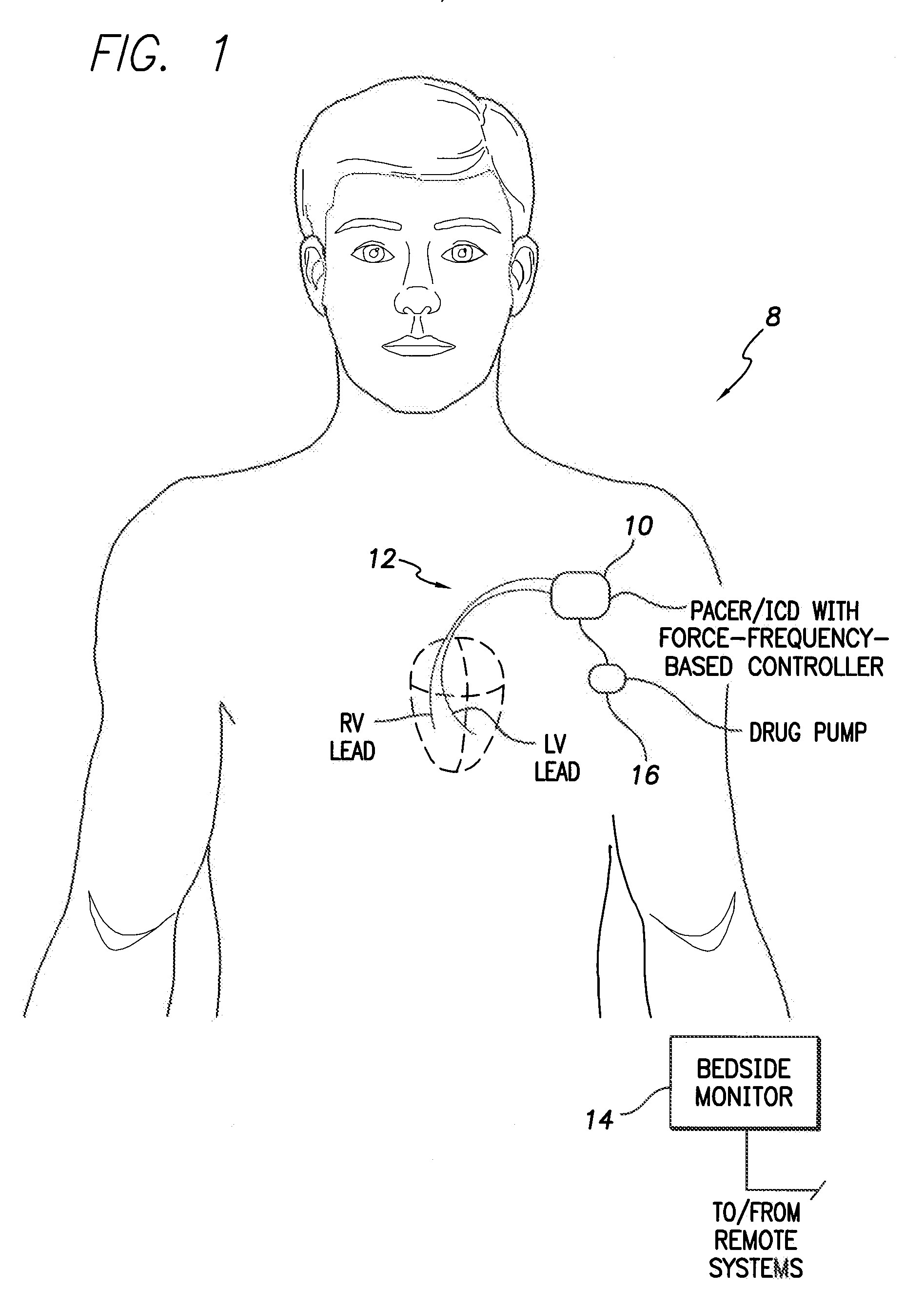
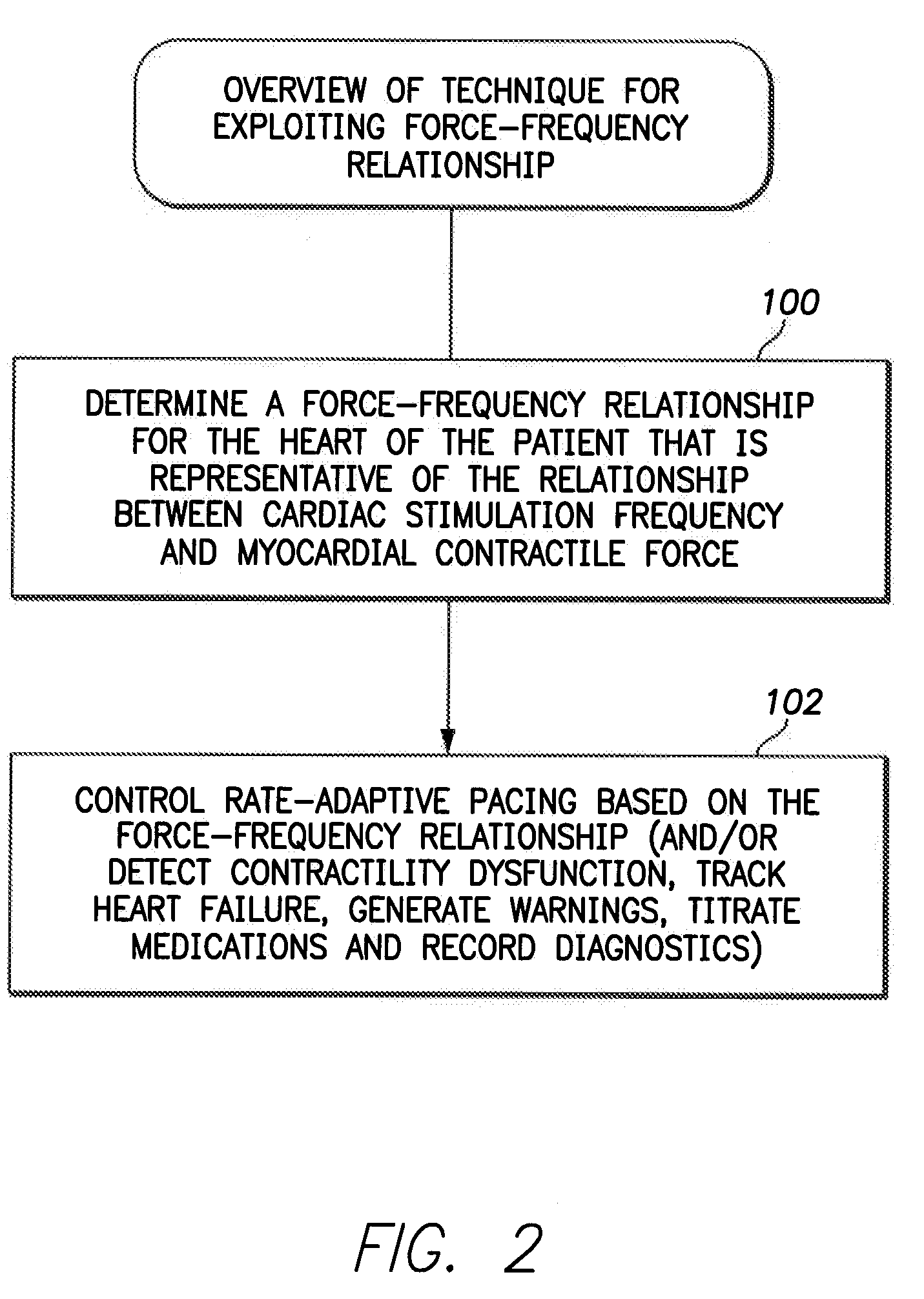
System and method for controlling rate-adaptive pacing based on a cardiac force-frequency relation detected by an implantable medical device
InactiveUS20100234906A1Reduce slopeDecrease in abscissaCatheterHeart stimulatorsCardiac pacemaker electrodeImplantable cardioverter-defibrillator
Techniques are provided for use in controlling rate-adaptive pacing within implantable medical devices such as pacemakers or implantable cardioverter-defibrillators (ICDs). In one example, a force-frequency relationship is determined for the heart of the patient, which is representative of the relationship between cardiac stimulation frequency and myocardial contractile force. To this end, various parameters are detected for use as surrogates for contractile force, including selected systolic pressure parameters and cardiogenic impedance parameters. Rate-adaptive pacing is then controlled based on the detected force-frequency relationship to, for example, deactivate rate-adaptive pacing if the slope and / or abscissa of the force-frequency relationship indicates significant contractility dysfunction within the patient. In other examples, rather than deactivating rate-adaptive pacing, control parameters are adjusted to render the rate-adaptive pacing less aggressive. In still other examples, trends in the slope and / or abscissa of the force-frequency relationship are monitored to detect contractility dysfunction and / or heart failure and titrate medications accordingly.
Owner:PACESETTER INC
Leadless intra-cardiac medical device with dual chamber sensing through electrical and/or mechanical sensing
ActiveUS20130325081A1Small sizeConvenient to accommodateHeart stimulatorsDiagnostic recording/measuringCardiac activityCardiac chamber
A leadless intra-cardiac medical device senses cardiac activity from multiple chambers and applies cardiac stimulation to at least one cardiac chamber and / or generates a cardiac diagnostic indication. The leadless device may be implanted in a local cardiac chamber (e.g., the right ventricle) and detect near-field signals from that chamber as well as far-field signals from an adjacent chamber (e.g., the right atrium).
Owner:PACESETTER INC
Method and apparatus for optimizing cardiac resynchronization therapy based on left ventricular acceleration
InactiveUS6885889B2Good effectExtension of timeHeart stimulatorsLeft cardiac chamberLeft ventricular size
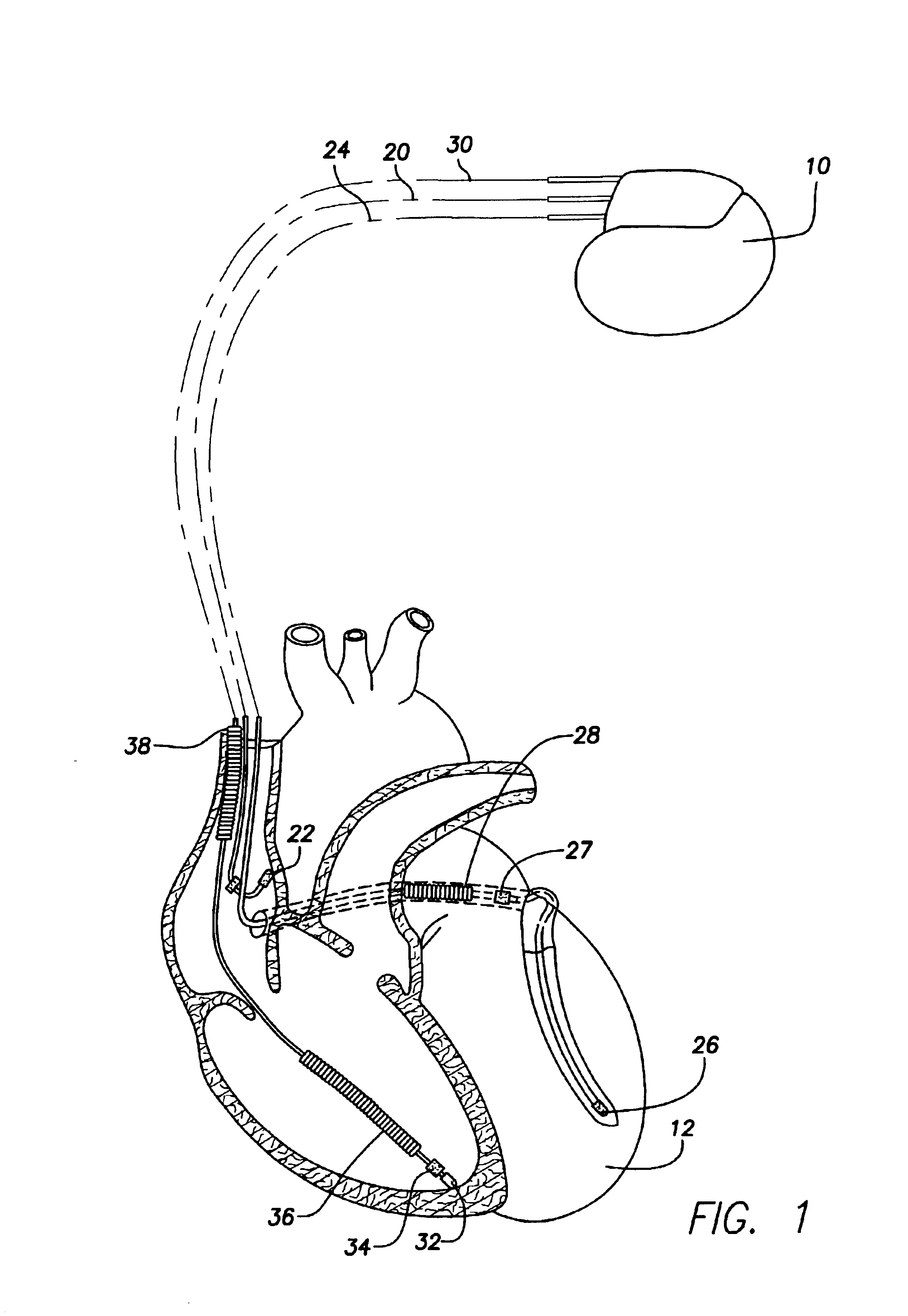
A system and method for monitoring left ventricular cardiac contractility and for optimizing a cardiac therapy based on left ventricular lateral wall acceleration (LVA) are provided. The system includes an implantable or external cardiac stimulation device in association with a set of leads including a left ventricular epicardial or coronary sinus lead equipped with an acceleration sensor. The device receives and processes acceleration sensor signals to determine a signal characteristic indicative of LVA during isovolumic contraction. A therapy optimization method evaluates the LVA during varying therapy settings and selects the setting(s) that correspond to a maximum LVA during isovolumic contraction. In one embodiment, the optimal inter-ventricular pacing interval for use in cardiac resynchronization therapy is determined as the interval corresponding to the highest amplitude of the first LVA peak during isovolumic contraction.
Owner:MEDTRONIC INC
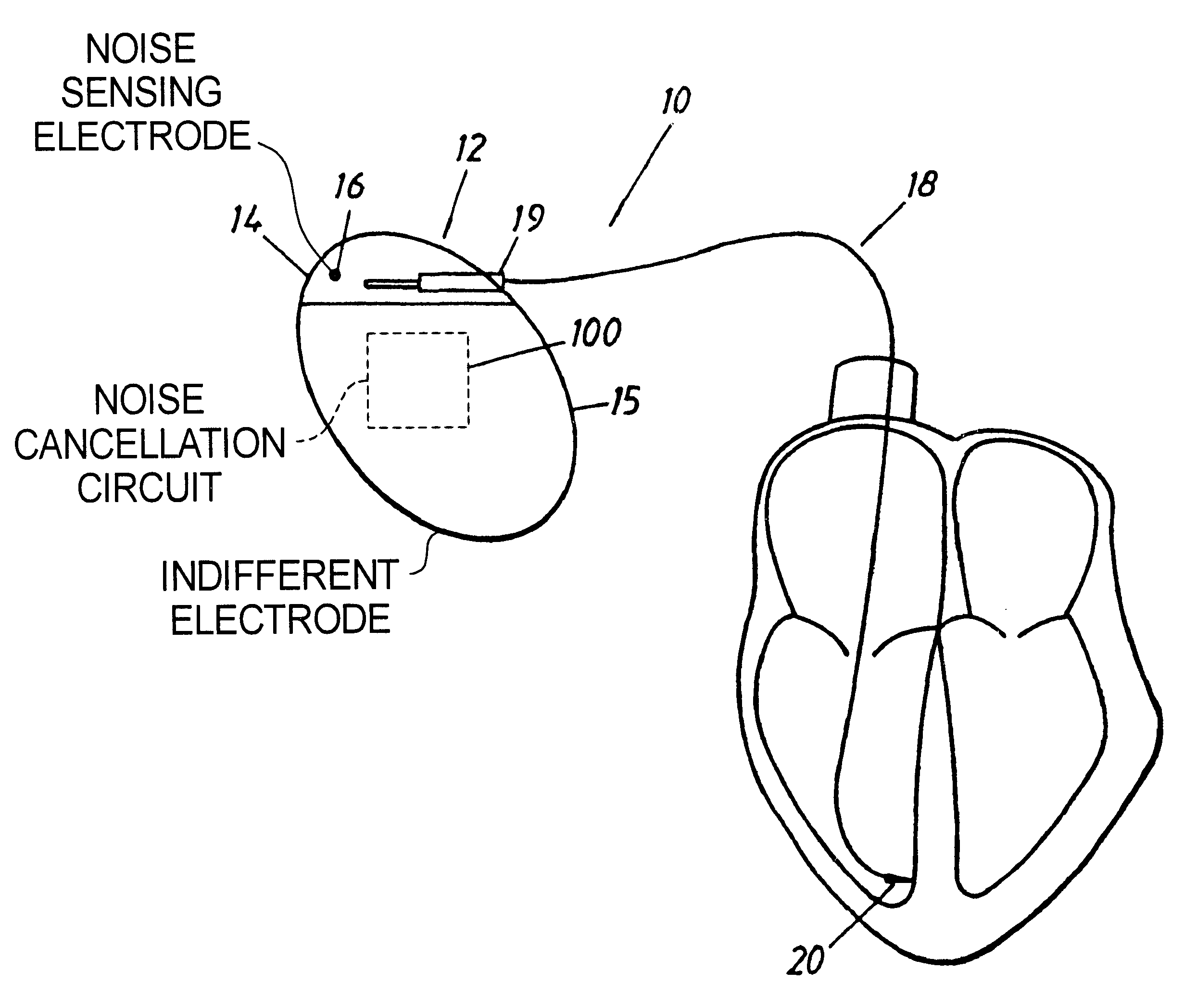
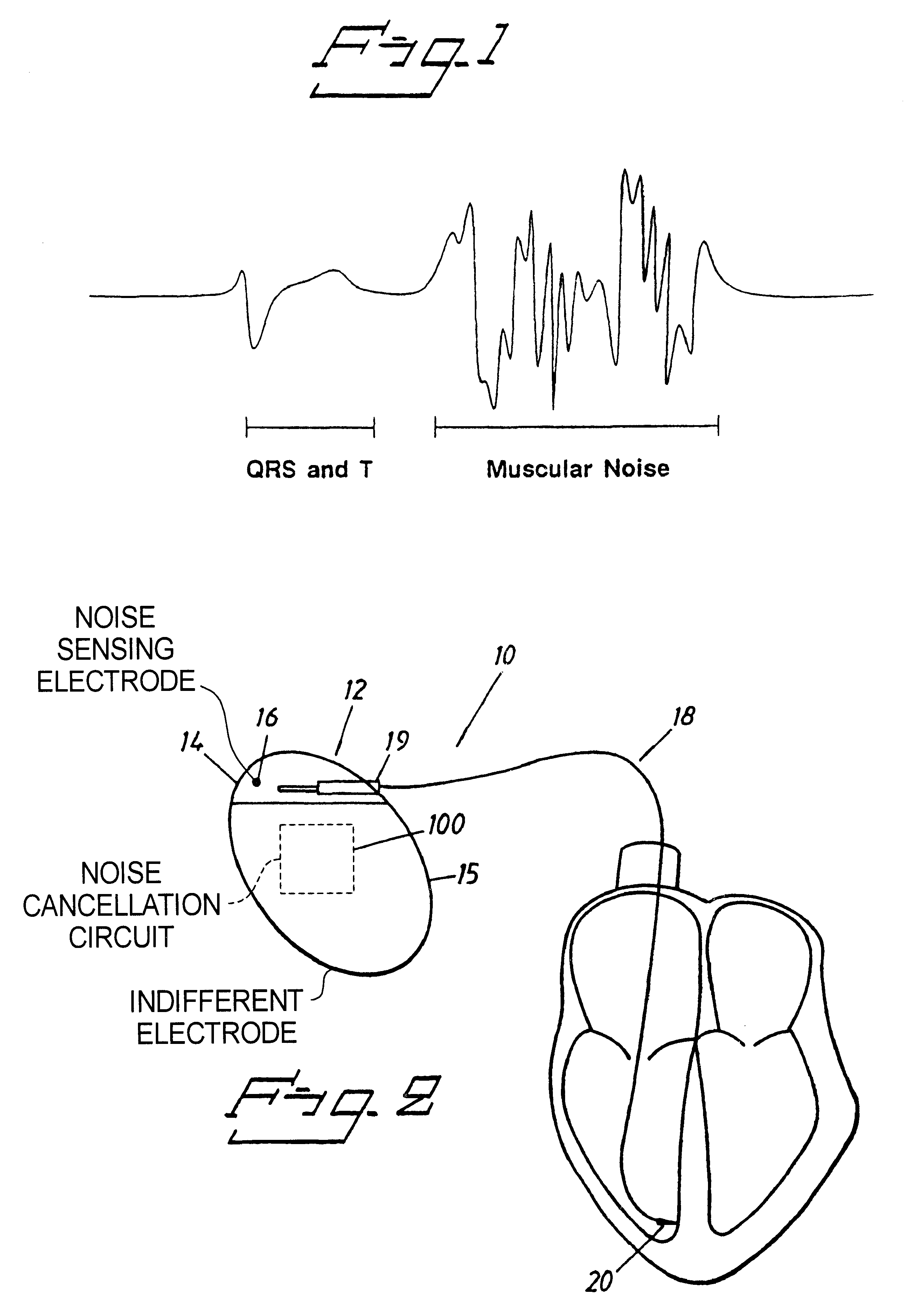
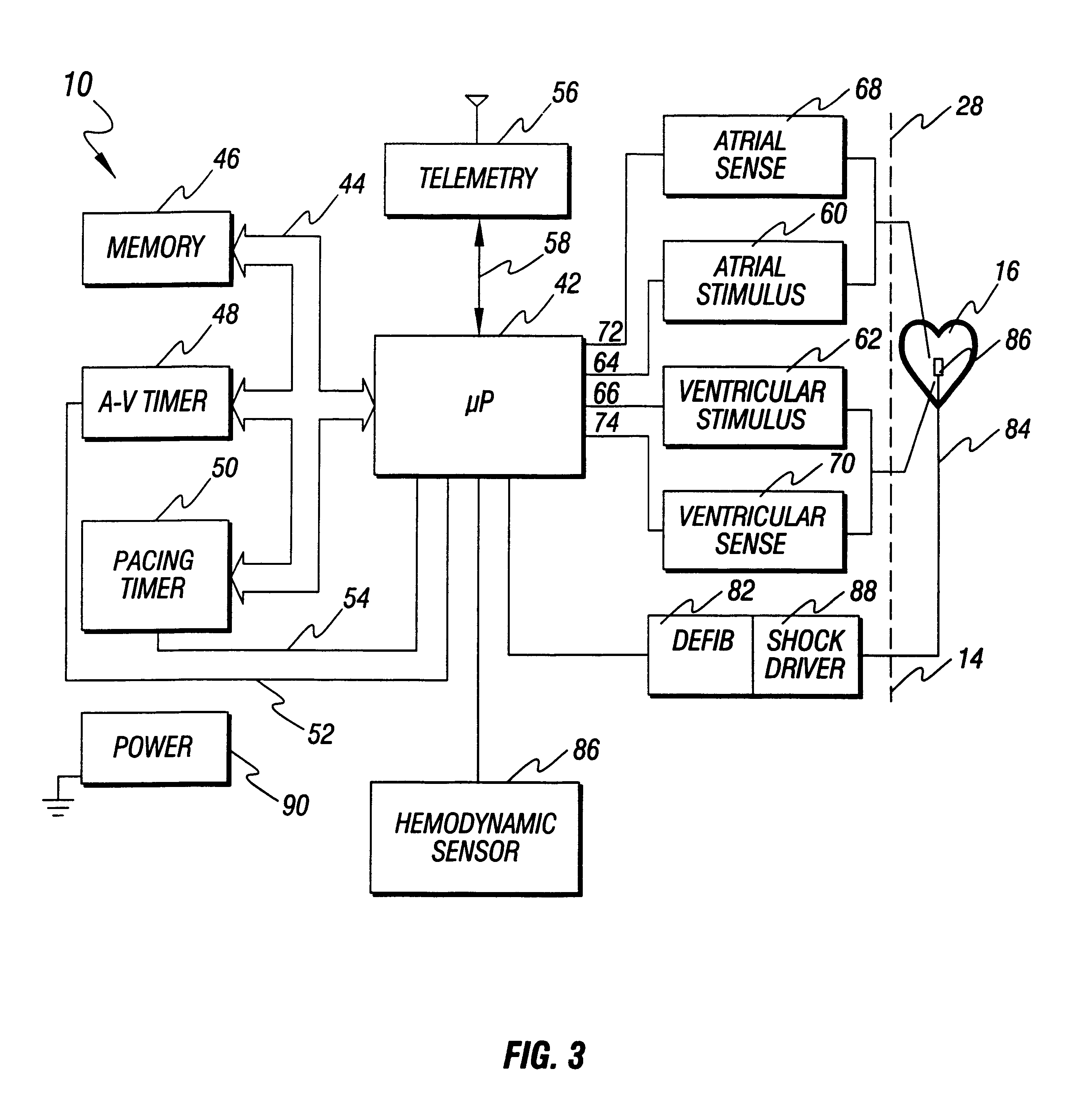
Implantable cardiac stimulator with circuitry for removing noise in sensed electrical signals
An implantable medical device, such as a cardiac stimulator, has a noise cancelling circuit which cancels noise signals relating to body movements which originate outside of the heart, and which are sensed between a noise sensing electrode located outside of the heart and the indifferent electrode of the stimulator housing. The noise cancelling circuit cancels these noise signals from the electrical signals which originate within the heart and which are sensed between the tip electrode of a stimulator lead and the indifferent electrode of the stimulator housing.
Owner:PACESETTER AB
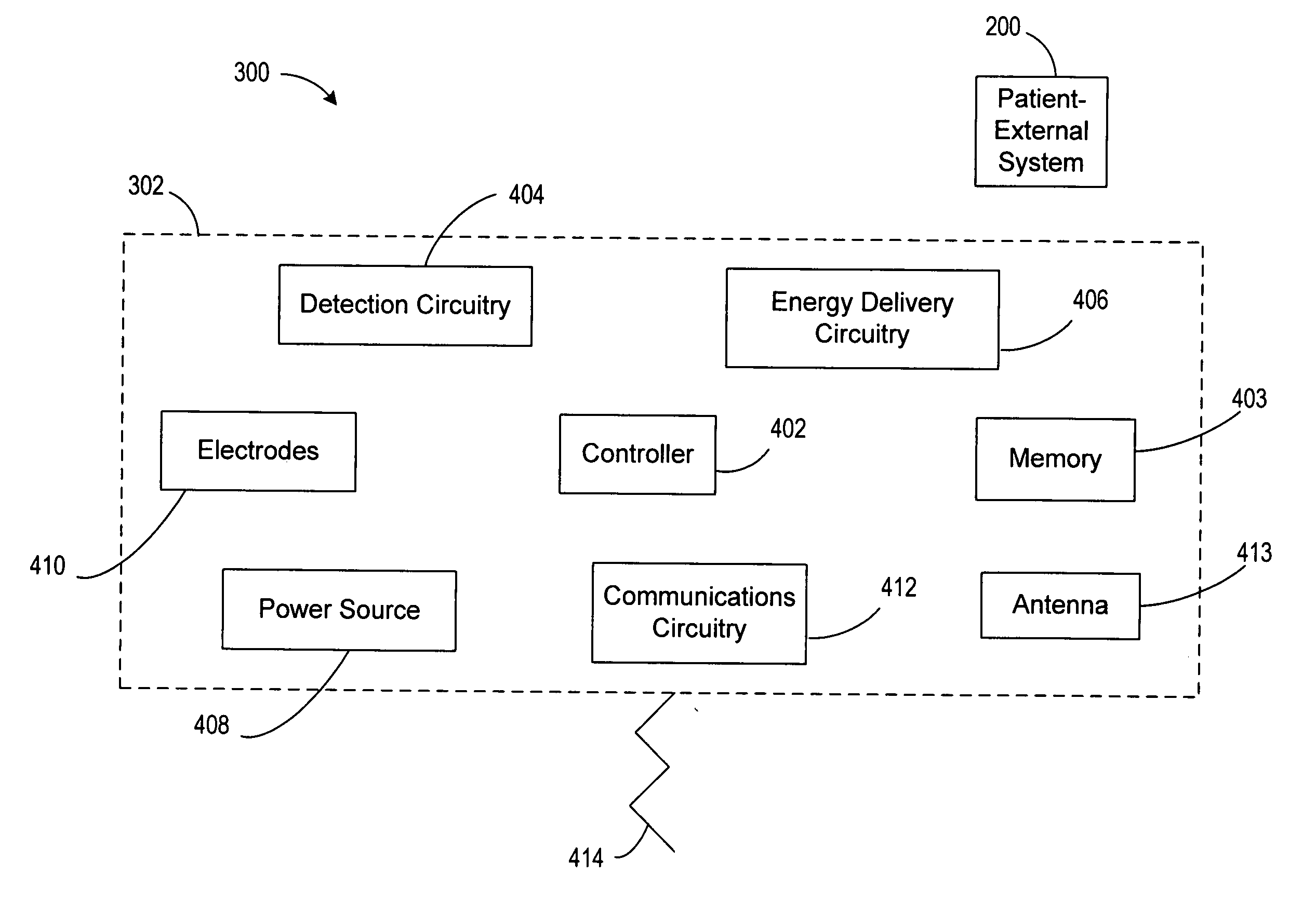
Leadless cardiac stimulation device employing distributed logic
ActiveUS20060135999A1Epicardial electrodesTransvascular endocardial electrodesCardiac activityThoracic cavity
Systems and methods involve an intrathoracic cardiac stimulation device operable to provide autonomous cardiac sensing and energy delivery. The cardiac stimulation device includes a housing configured for intrathoracic placement relative to a patient's heart. A fixation arrangement of the housing is configured to affix the housing at an implant location within cardiac tissue or cardiac vasculature. An electrode arrangement supported by the housing is configured to sense cardiac activity and deliver stimulation energy to the cardiac tissue or cardiac vasculature. Energy delivery circuitry in the housing is coupled to the electrode arrangement. Detection circuitry is provided in the housing and coupled to the electrode arrangement. Communications circuitry may optionally be supported by the housing. A controller in the housing coordinates delivery of energy to the cardiac tissue or cardiac vasculature in accordance with an energy delivery protocol appropriate for the implant location.
Owner:CARDIAC PACEMAKERS INC
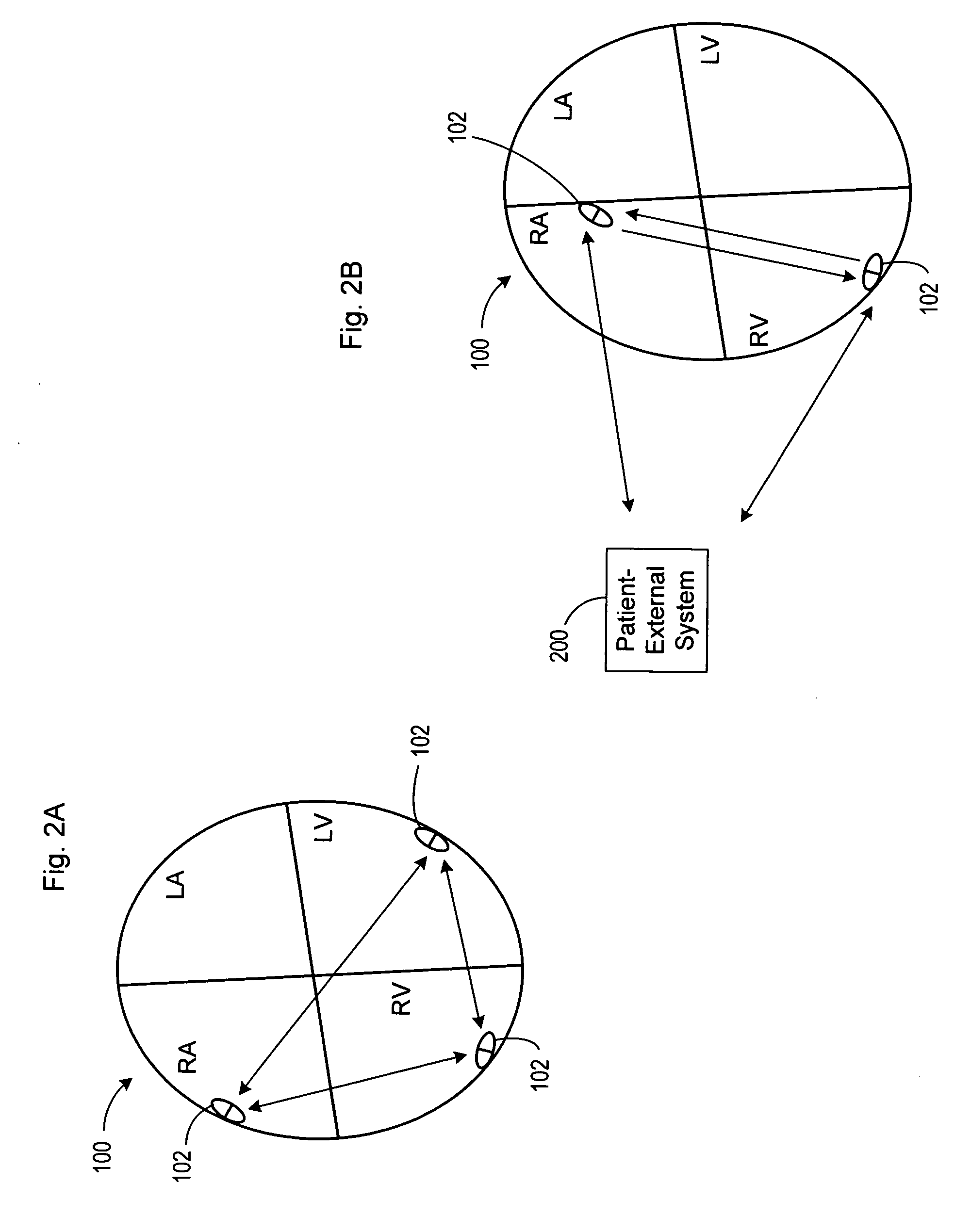
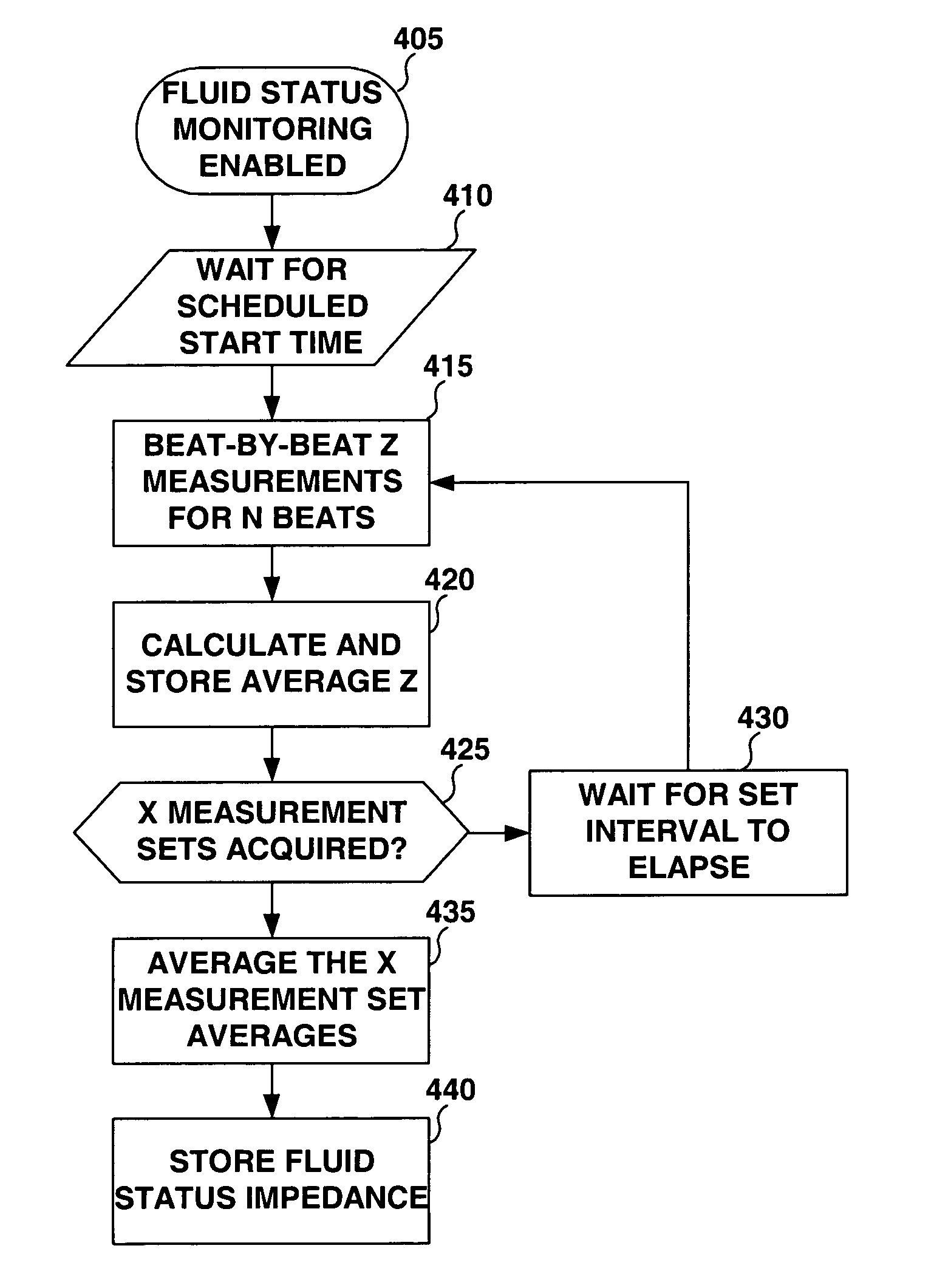
Method and apparatus for monitoring tissue fluid content for use in an implantable cardiac device
ActiveUS20050080460A1Easy to implementCancel noiseHeart defibrillatorsHeart stimulatorsThoracic FluidTissue fluid
Owner:MEDTRONIC INC
Cardiac stimulation device for optimizing cardiac output with myocardial ischemia protection
InactiveUS6865420B1Reduce demandReduce detectionHeart stimulatorsCardiac muscleIntracardiac Electrogram
A cardiac stimulation device and method detect myocardial ischemia and provide a response for alleviating the ischemia. Myocardial ischemia is detected by identifying changes in the ST-segment of the intracardiac electrogram (EGM) sensed using large sensing electrode surfaces created by electrically coupling one or more cardiac electrodes or by using larger surface area shocking coils. Myocardial ischemia monitoring is performed when stimulation parameters are adjusted for increasing cardiac output, causing an increased metabolic demand to be placed on the myocardium itself. When myocardial ischemia is detected, stimulation parameters are re-adjusted to reduce the demand placed on the myocardium and thereby alleviate the ischemia.
Owner:PACESETTER INC
Cardiac stimulation device including sleep apnea prevention and treatment
InactiveUS7212862B2Prevents sleep apneaReduce outputHeart stimulatorsArtificial respirationTreatment sleepPulse rate
An implantable cardiac stimulation device comprises a physiologic sensor and one or more pulse generators. The physiologic sensor is capable of sensing a physiologic parameter. The pulse generators can generate cardiac pacing pulses with a timing based on the physiologic parameter. The timed cardiac pacing pulses can prevent a sleep apnea condition. In one example, a cardiac stimulation device has a physiologic sensor and can be configured to pace a patient's heart according to a rest mode of operation. The cardiac stimulation device uses measurements from the physiologic sensor to prevent and treat sleep apnea using a revised rest mode of operation. The revised rest mode operates under a presumption that sleep apnea is primary to a reduced heart rate, rather than secondary, so that pacing at a rate higher than the natural cardiac rate during sleep will prevent sleep apnea.
Owner:PACESETTER INC
Sleep apnea therapy device using dynamic overdrive pacing
A cardiac stimulation device uses dynamic overdrive pacing to prevent sleep apnea. In another aspect, the device can use dynamic overdrive pacing to terminate sleep apnea after detection. An implantable cardiac stimulation device comprises a sensor and one or more pulse generators. The sensor senses intrinsic cardiac electrical phenomena. The pulse generators can generate cardiac pacing pulses with timing based on the sensed intrinsic cardiac electrical phenomena to dynamically overdrive the intrinsic cardiac electrical phenomena. The timed cardiac pacing pulses can prevent a sleep apnea condition.
Owner:PACESETTER INC
Cardiac stimulation device including sleep apnea prevention and treatment
InactiveUS6999817B2Prevents sleep apneaAvoid adjustmentHeart stimulatorsArtificial respirationTreatment sleepPulse rate
An implantable cardiac stimulation device comprises a physiologic sensor and one or more pulse generators. The physiologic sensor is capable of sensing a physiologic parameter. The pulse generators can generate cardiac pacing pulses with a timing based on the physiologic parameter. The timed cardiac pacing pulses can prevent a sleep apnea condition. In one example, a cardiac stimulation device has a physiologic sensor and can be configured to pace a patient's heart according to a rest mode of operation. The cardiac stimulation device uses measurements from the physiologic sensor to prevent and treat sleep apnea using a revised rest mode of operation. The revised rest mode operates under a presumption that sleep apnea is primary to a reduced heart rate, rather than secondary, so that pacing at a rate higher than the natural cardiac rate during sleep will prevent sleep apnea.
Owner:PACESETTER INC
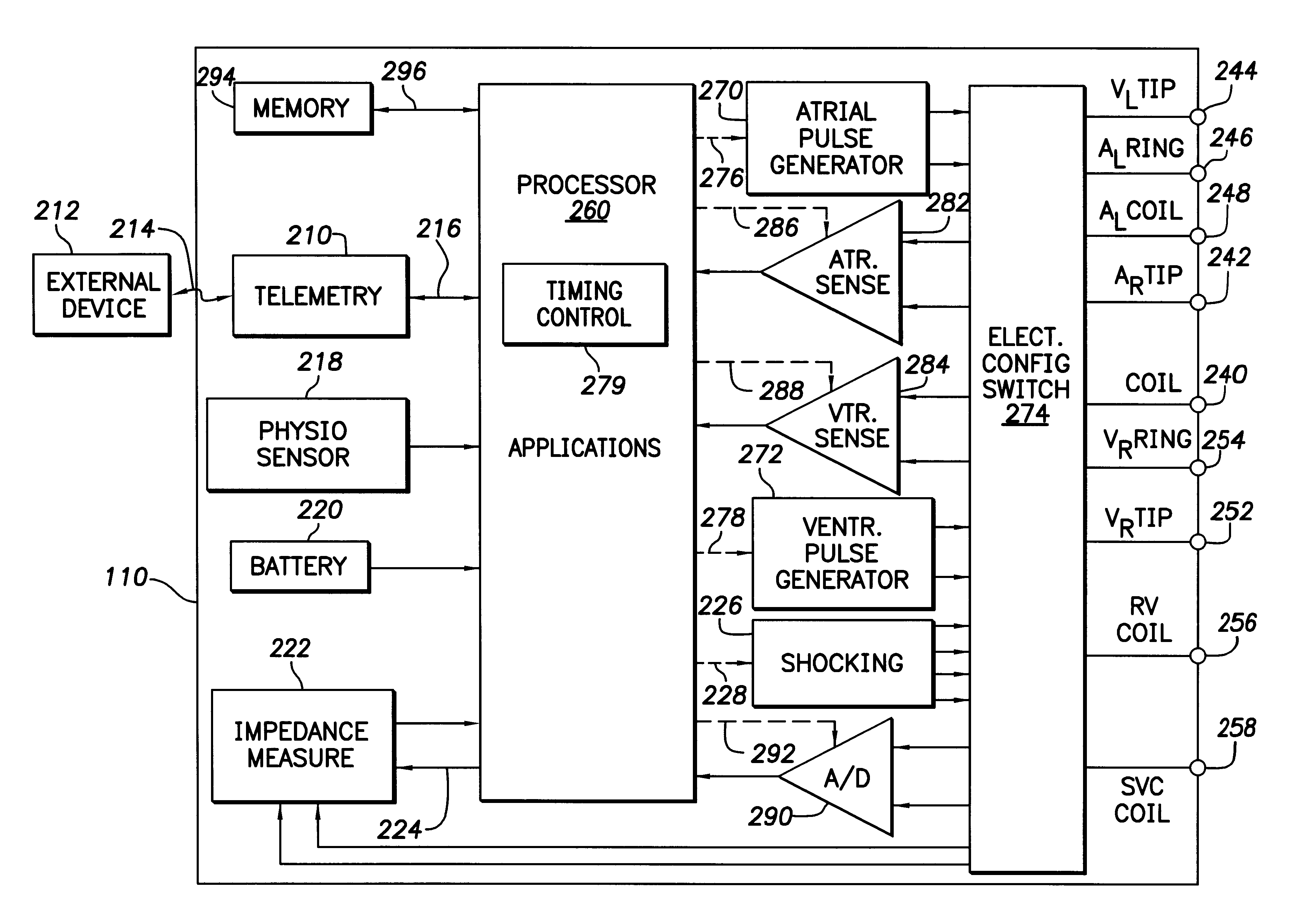
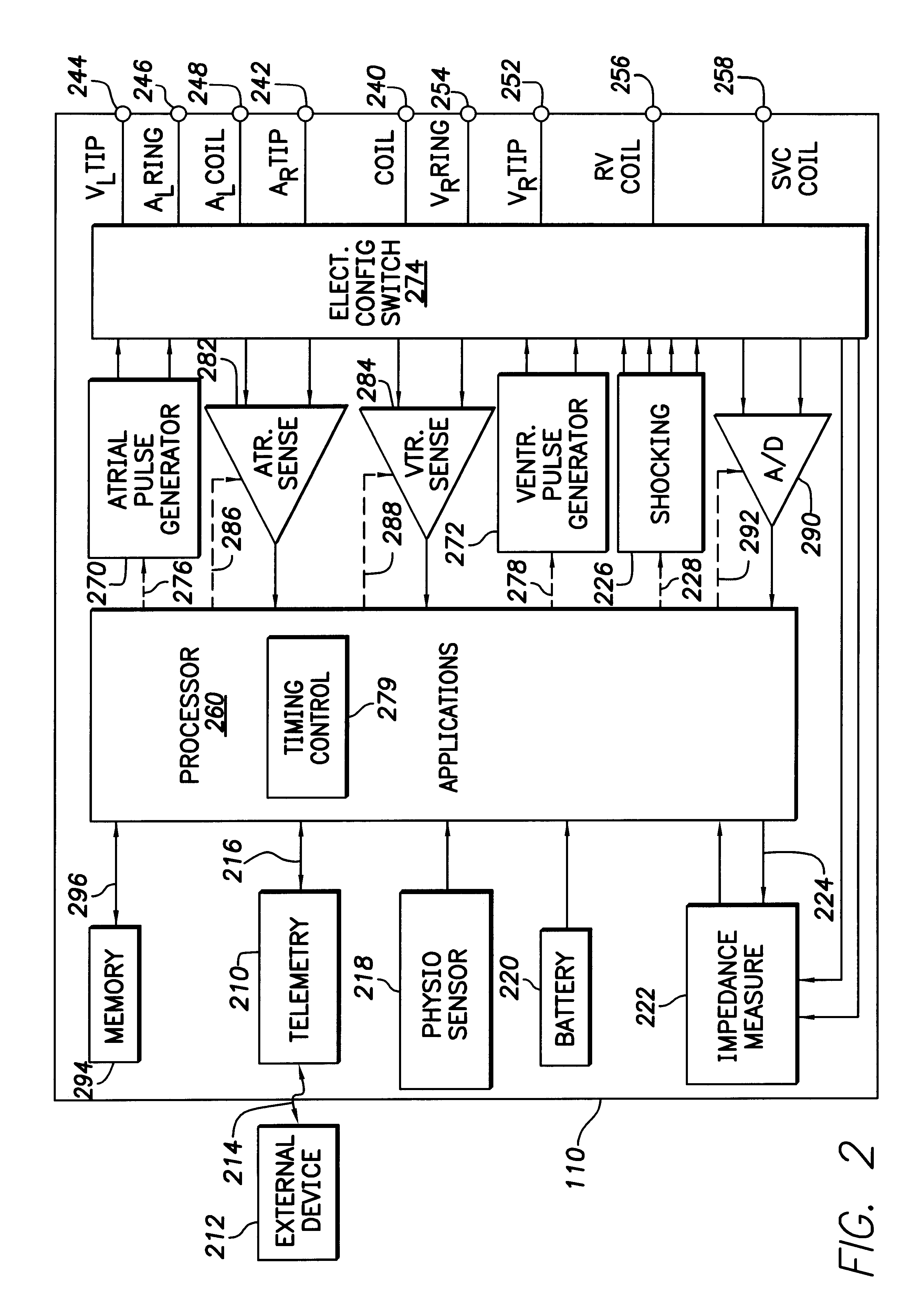
System and method for automatically selecting electrode polarity during sensing and stimulation
InactiveUS6477417B1Improve welfareImprove coordinationHeart defibrillatorsHeart stimulatorsElectricityElectrical battery
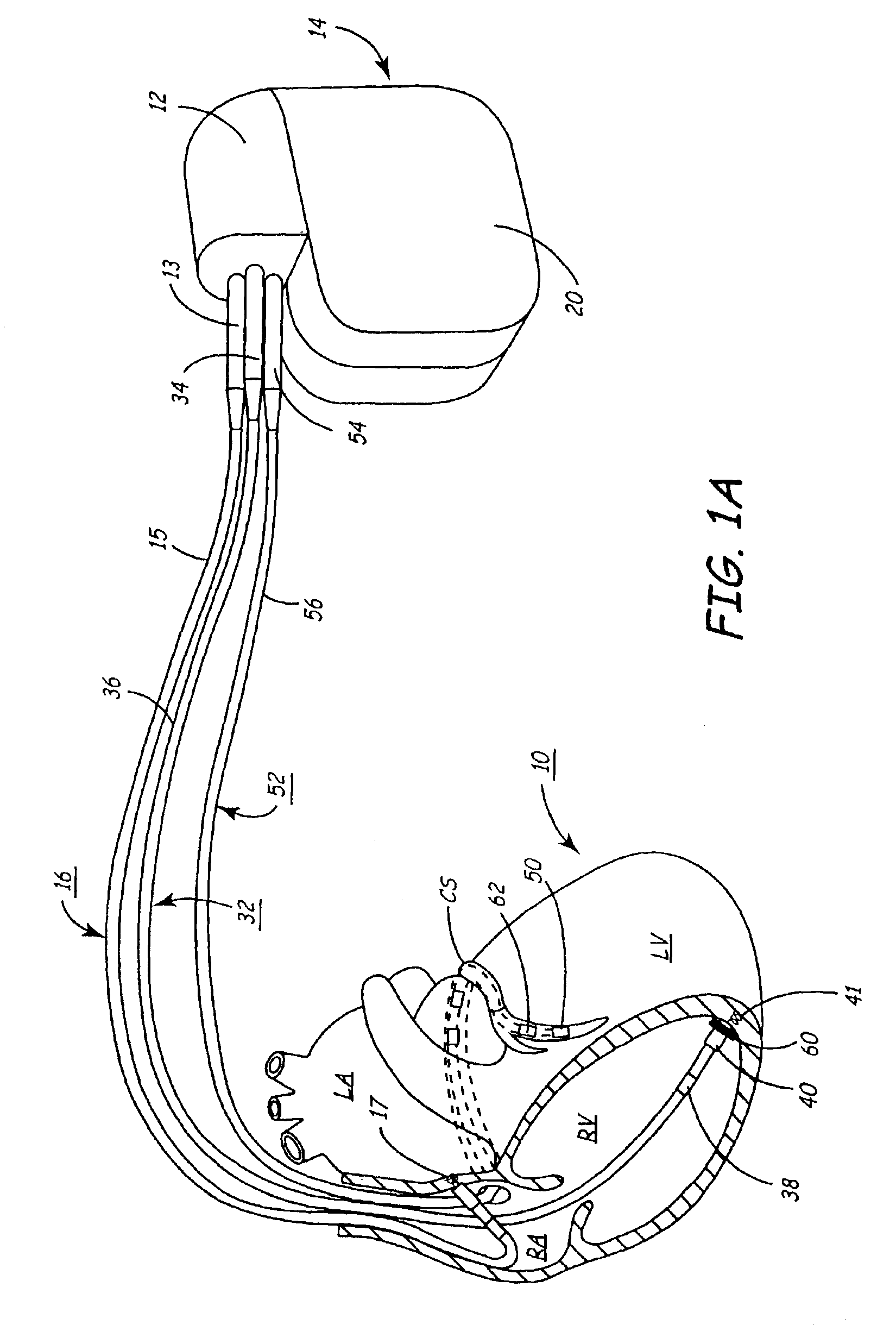
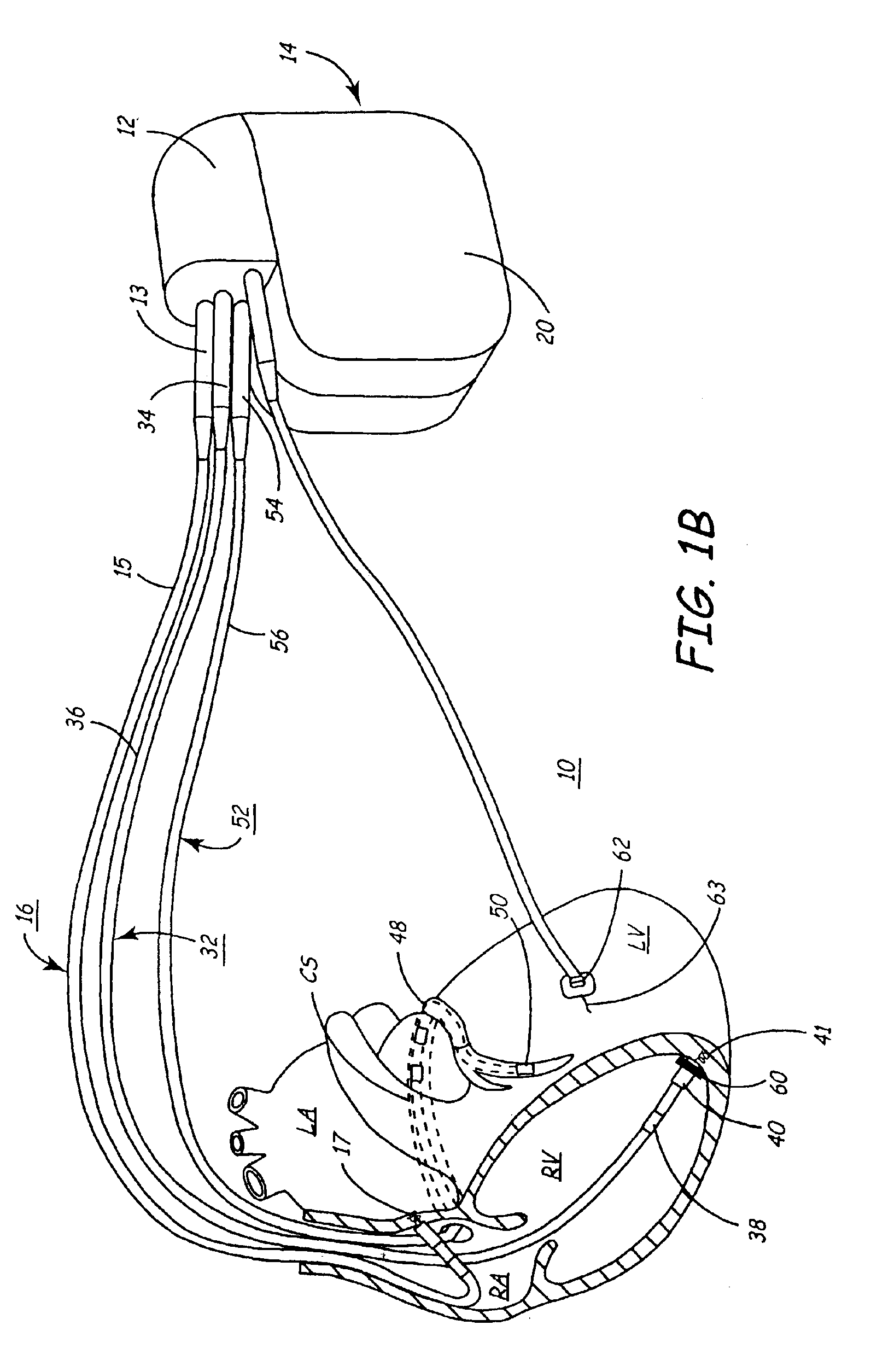
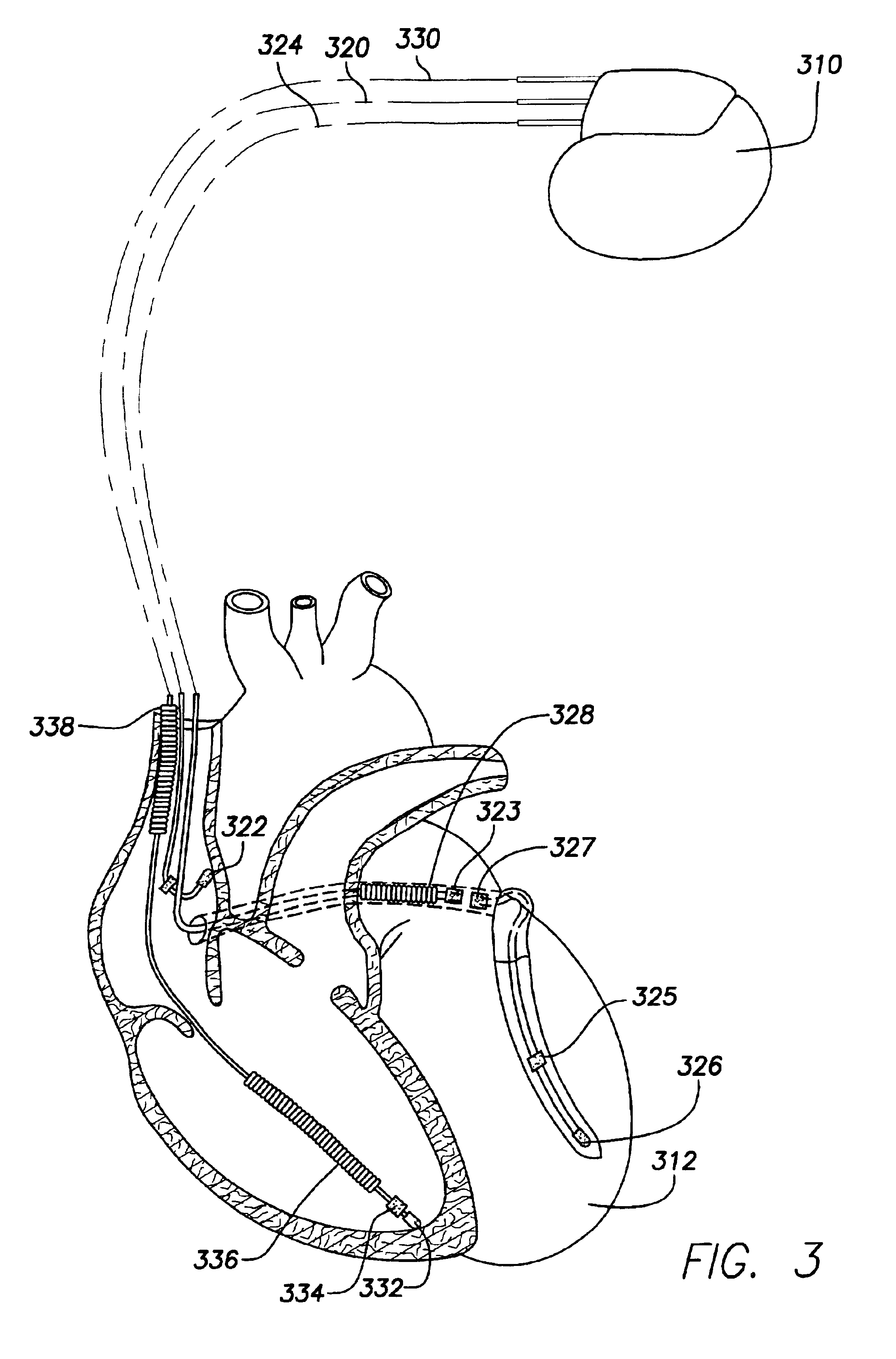
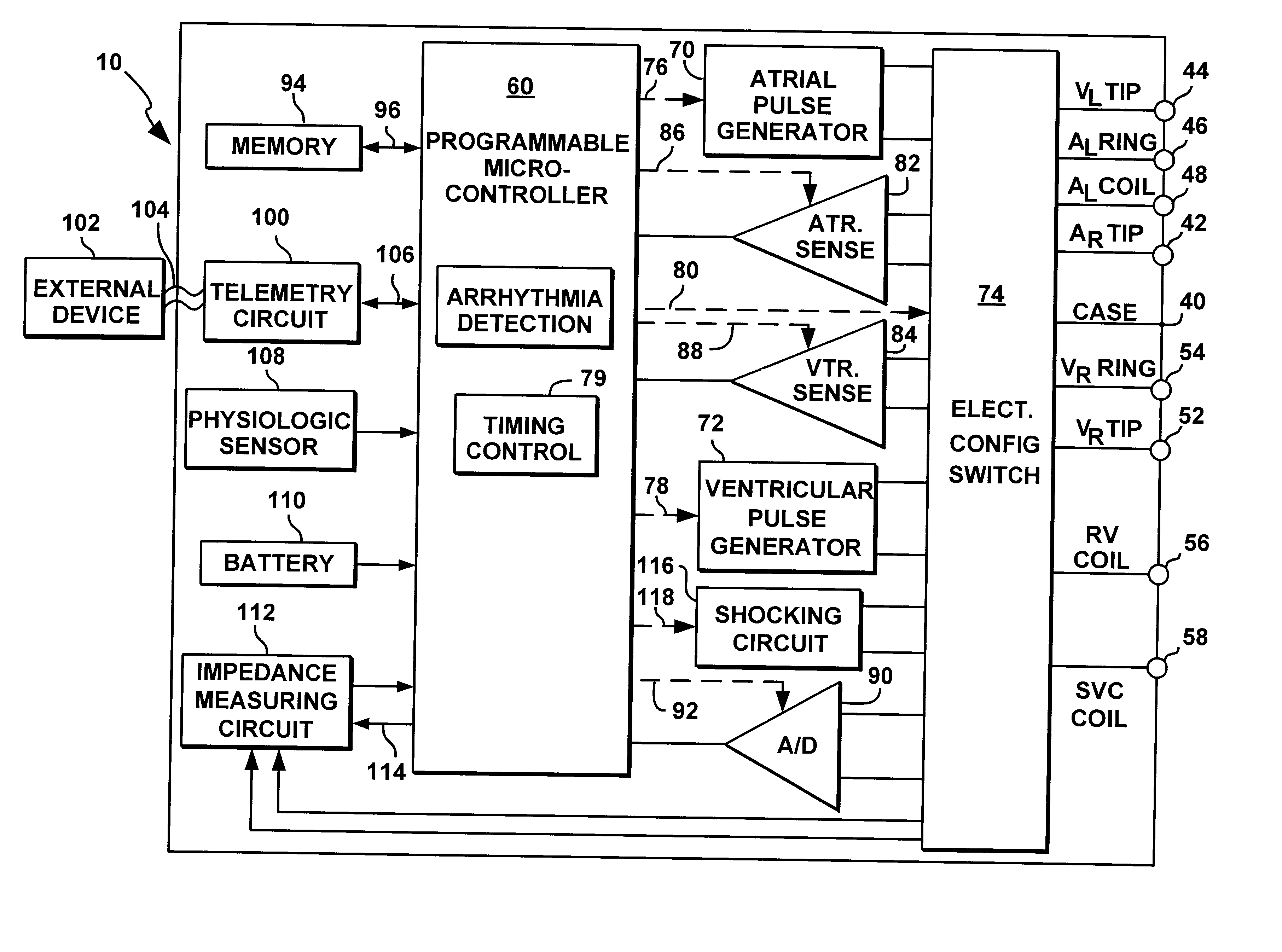
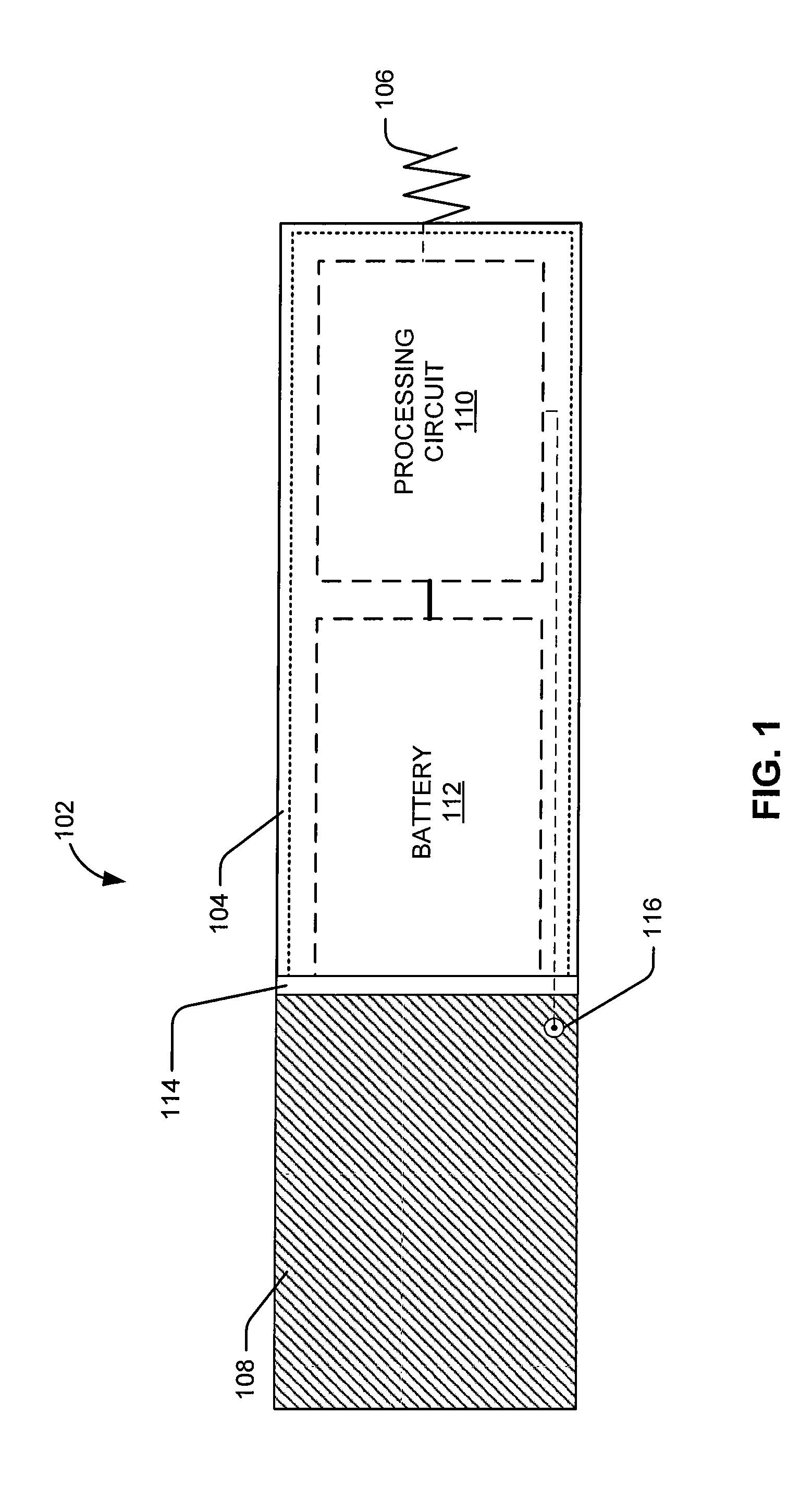
An implantable multi-chamber cardiac stimulation device includes flexibly programmable electrode stimulation configurations, and is capable of precisely controlling the stimulation sequence between multiple sites. The stimulation device provides a plurality of connection ports that allow independent connection of each electrical lead associated with a particular stimulation site in the heart. Each connection port further provides a unique terminal for making electrical contact with only one electrode such that no two electrodes are required to be electrically coupled. Furthermore, each electrode, whether residing on a unipolar, bipolar or multipolar lead, may be selectively connected or disconnected through programmable switching circuitry that determines the electrode configurations to be used for sensing and for stimulating at each stimulation site. The stimulation device allows for the programmable selection of each electrode terminal connection to a relatively positive or negative battery potential. In this way, each electrode, when electrically connected, may be programmed to act as the cathode or as the anode during sensing or stimulation delivery. Thus, directionality of the depolarization wave may be controlled by programming the cathode and anode assignments of the stimulation electrodes.
Owner:PACESETTER INC
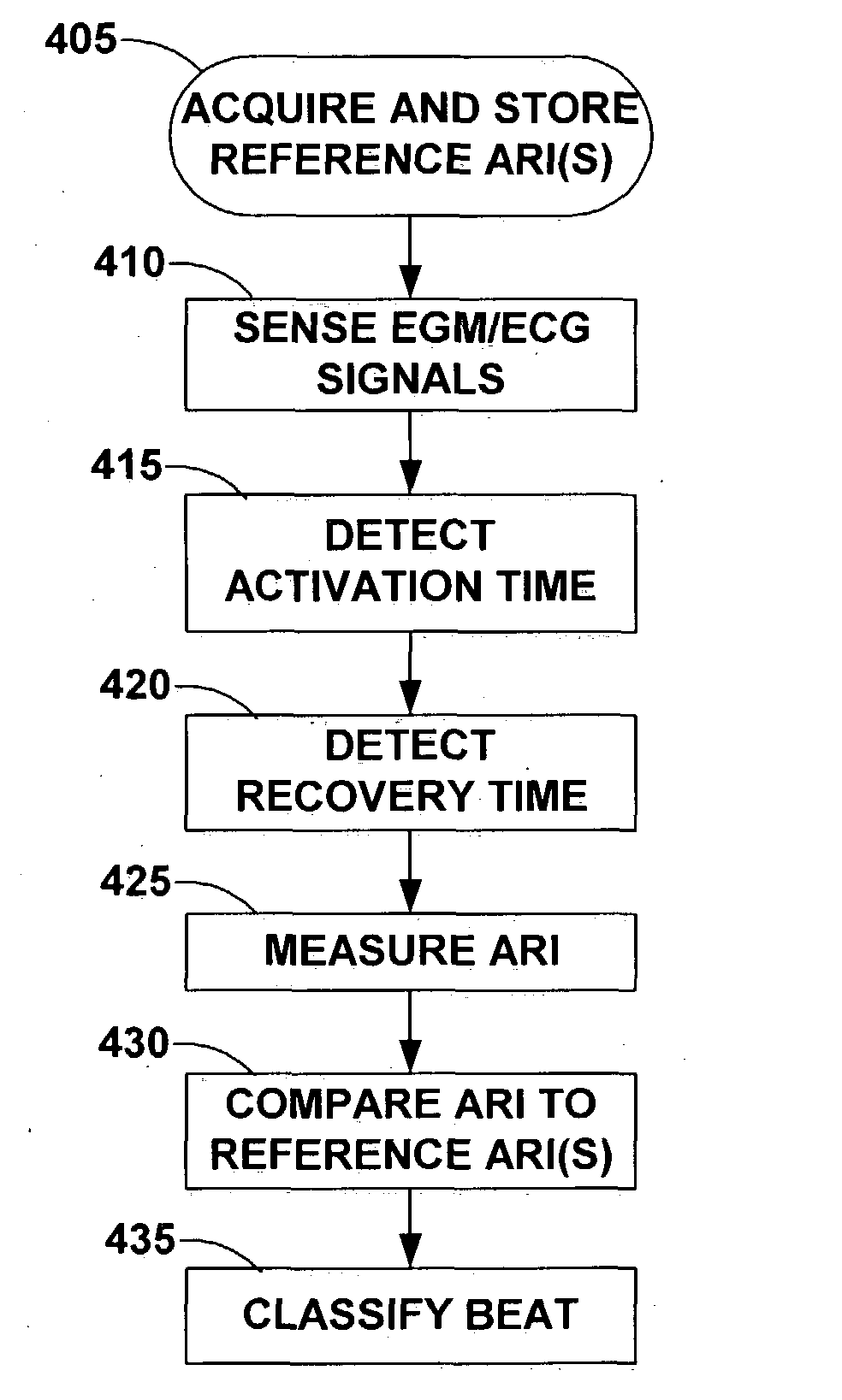
Activation recovery interval for classification of cardiac beats in an implanted device
The present invention provides a system and method for classifying cardiac beats based on activation-recovery intervals (ARIs) or an ARI-related parameter such as the spatial dispersion of activation, recovery or ARIs. The beat classification method may be used in monitoring and detecting cardiac rhythms and / or for controlling a cardiac stimulation therapy. The beat classification method includes acquiring a reference ARI for one or more known types of cardiac beats; measuring the activation-recovery interval of an unknown cardiac beat during cardiac activity monitoring; comparing the measured activation-recovery interval to the stored reference ARI(s); and classifying the cardiac beat based on the comparison between the measured ARI and the reference ARI(s).
Owner:MEDTRONIC INC
Cardiac pacing apparatus and method for continuous capture management
ActiveUS7831303B2Increase the number ofIncrease incidenceHeart stimulatorsHeart chamberCardiac stimulation
An implantable cardiac stimulation system and method having continuous capture management capabilities are provided. Continuous capture management is realized by continuously monitoring for secondary effects of loss of capture, thereby effectively providing continuous capture management in any heart chamber without encountering the limitations normally associated with evoked response sensing. A pacing threshold search is triggered upon detecting a secondary indicator of loss of capture. Secondary indicators of loss of capture may be lead-related changes, changes related to the occurrence of atrial sensed events, changes related related to the occurrence of ventricular sensed or paced events, and / or changes related to a monitored physiological condition.
Owner:MEDTRONIC INC
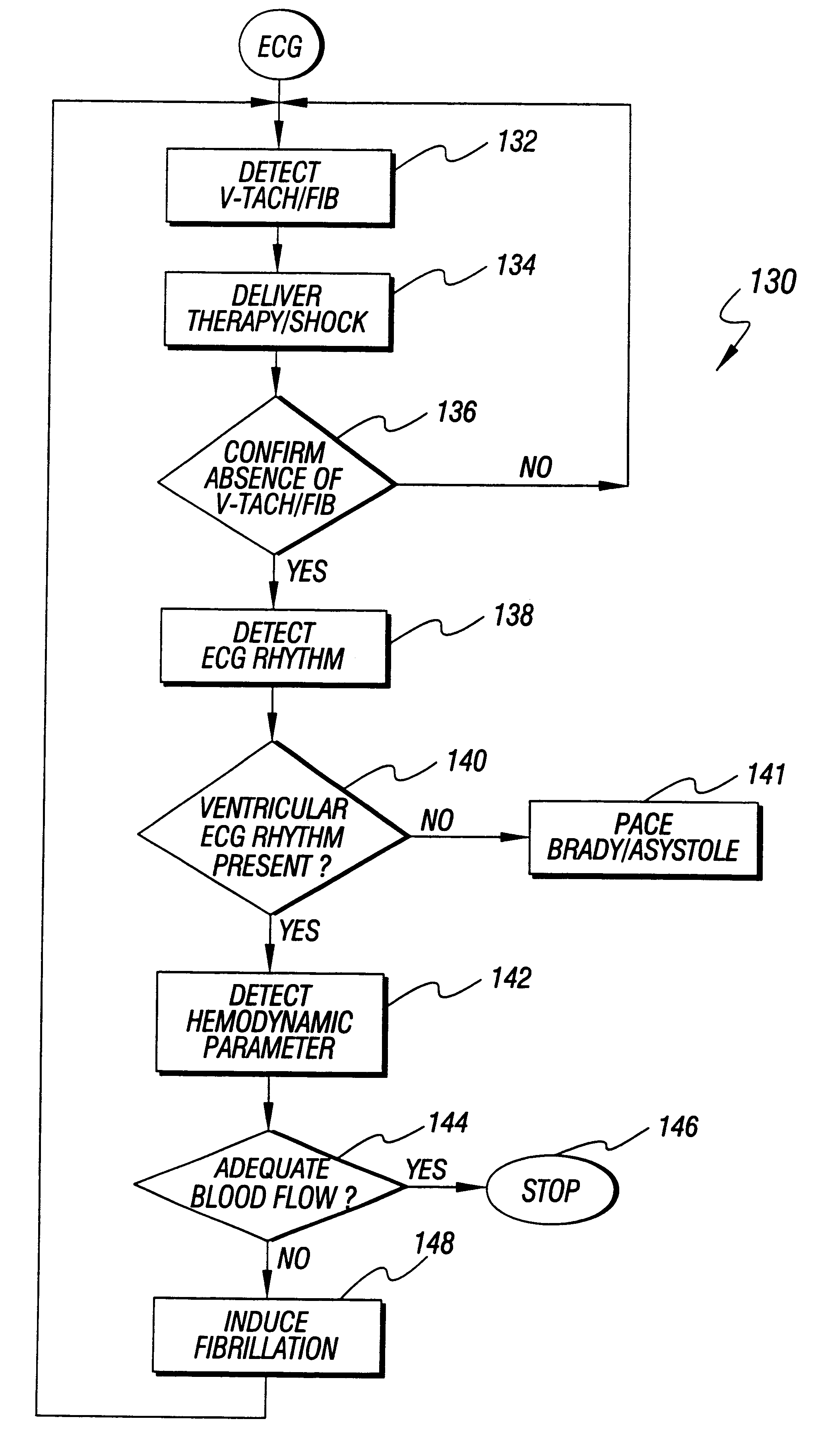
Method and apparatus for treatment of cardiac electromechanical dissociation
InactiveUS6298267B1Heart defibrillatorsCardiac arrest- pulseless electrical activityPulseless electrical activity
An apparatus and method for treating post-defibrillation electromechanical dissociation ("EMD") or pulseless electrical activity ("PEA"). A first embodiment comprises an implantable defibrillator with the capability of detecting and treating post defibrillation EMD. The stimulator / defibrillator has one or more leads with electrodes and at least one electrode for defibrillation. A sense circuit senses the electrical condition of the heart of the patient. A second sensor senses a parameter correlated to the state of blood flow. The cardiac stimulator / defibrillator detects and terminates ventricular tachyarrhythmia or fibrillation. If the stimulator / defibrillator detects the presence of electrical rhythm in the heart correlated, however, with inadequate blood flow to sustain life (EMD), the device provides an output to stimulate the heart to overcome EMD. The device may also be an external defibrillator. The method for treating the heart to restore blood flow where electromechanical dissociation occurs after termination of a ventricular tachyarrhythmia or ventricular fibrillation comprises identifying electromechanical disassociation after termination of a ventricular tachyarrhythmia or a fibrillation and inducing or re-inducing ventricular fibrillation and subsequently applying defibrillating shocks to terminate the fibrillation.
Owner:INTERMEDICS
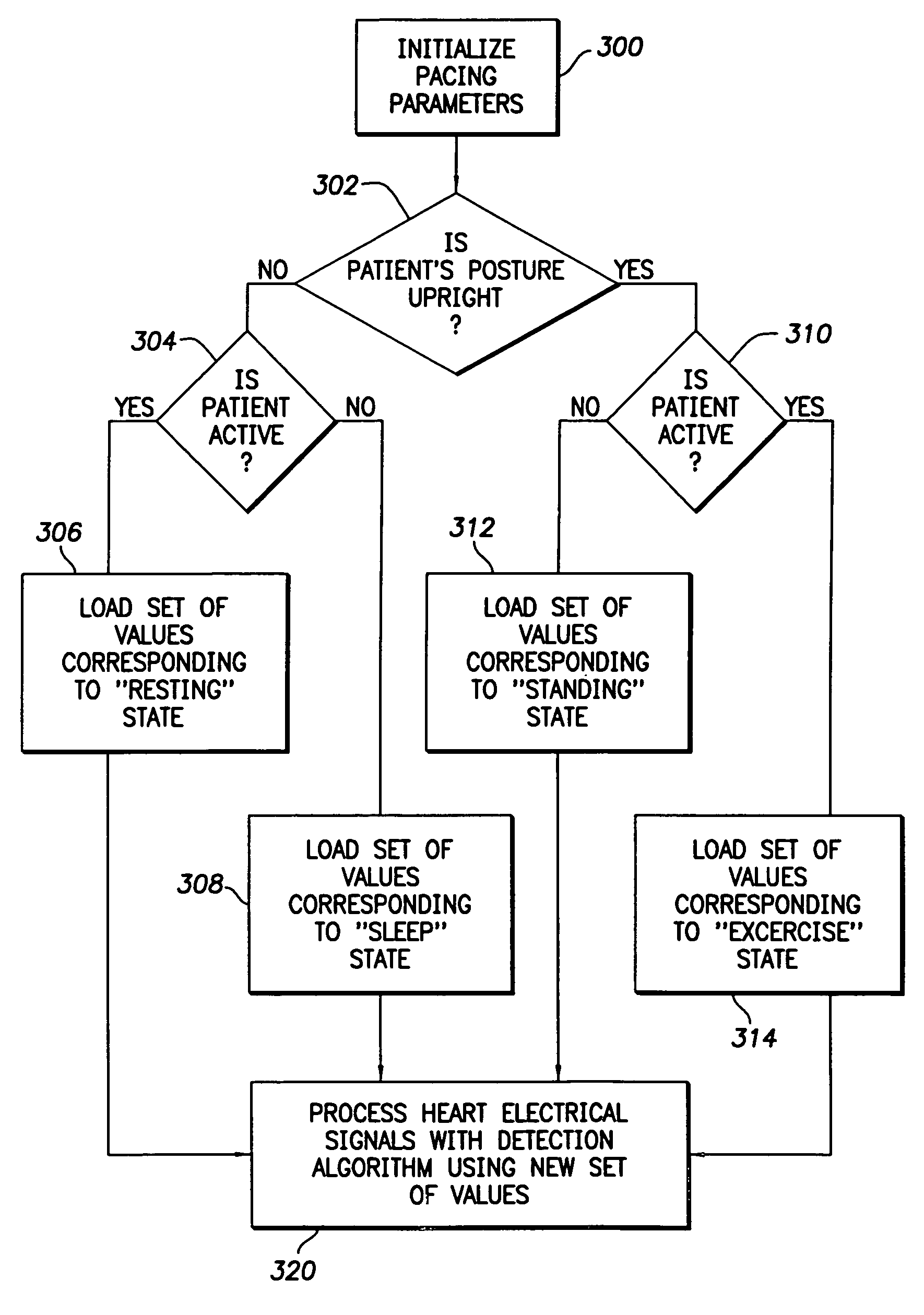
Modification of evoked response detection algorithm based on orientation and activity of patient
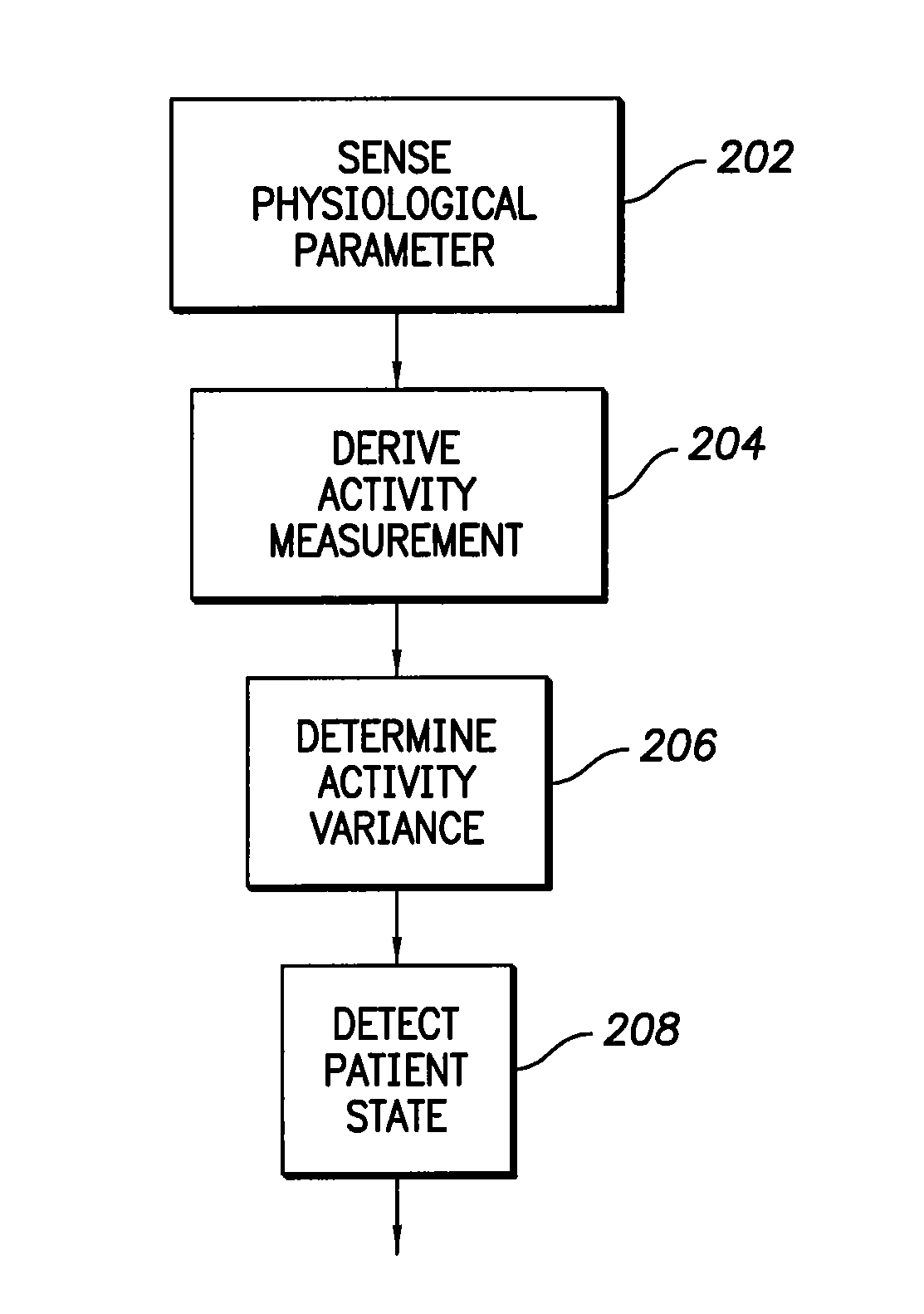
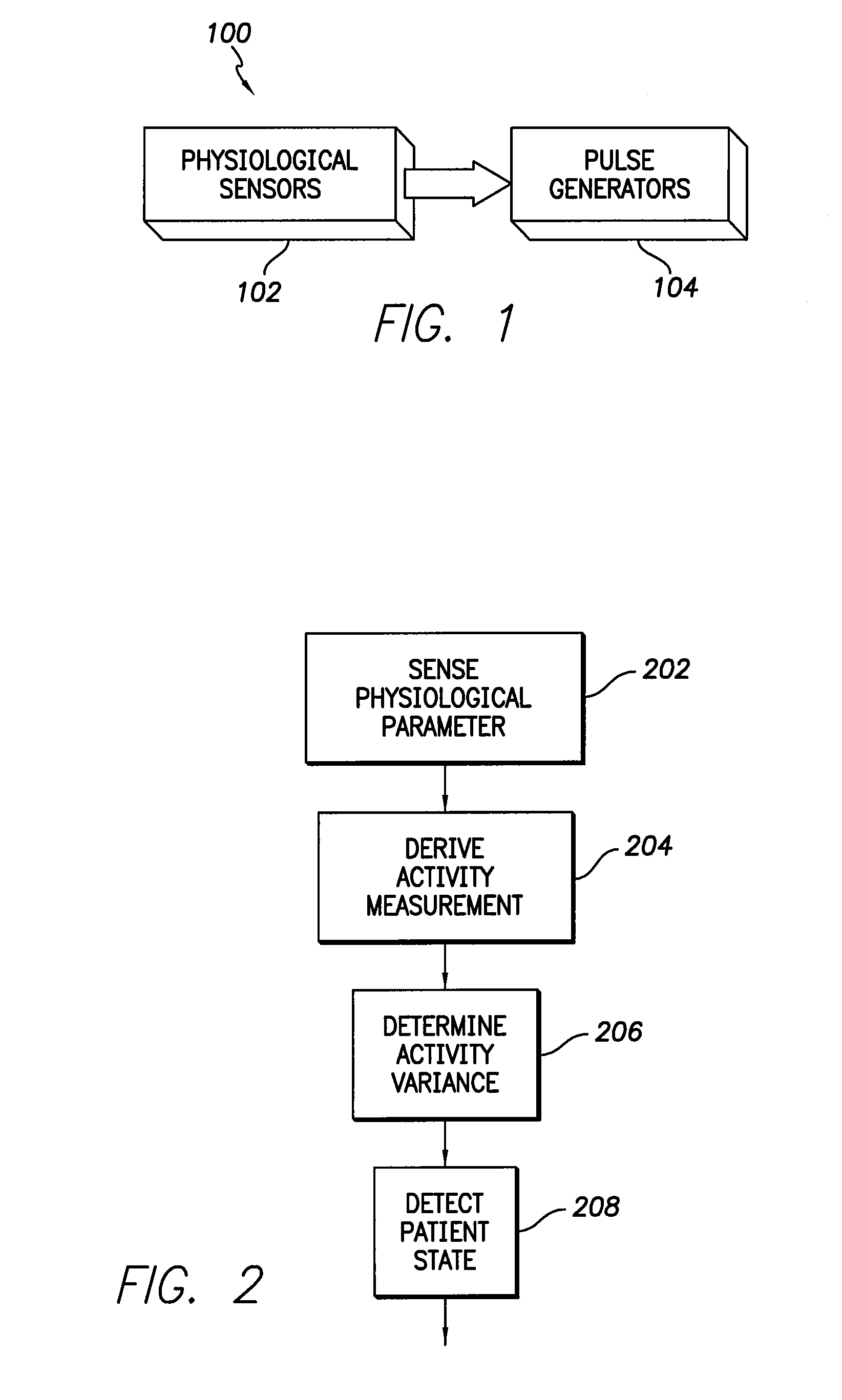
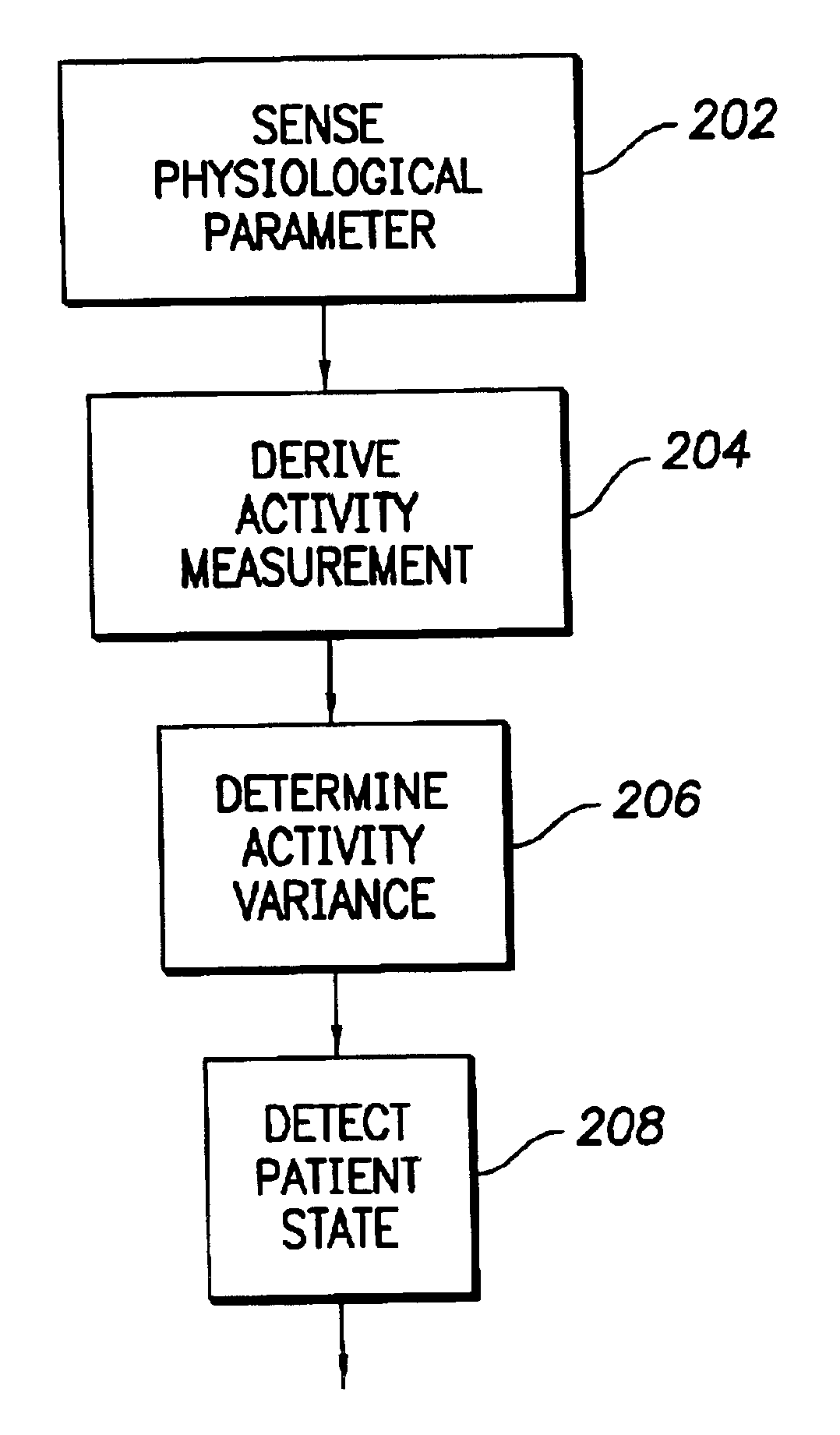
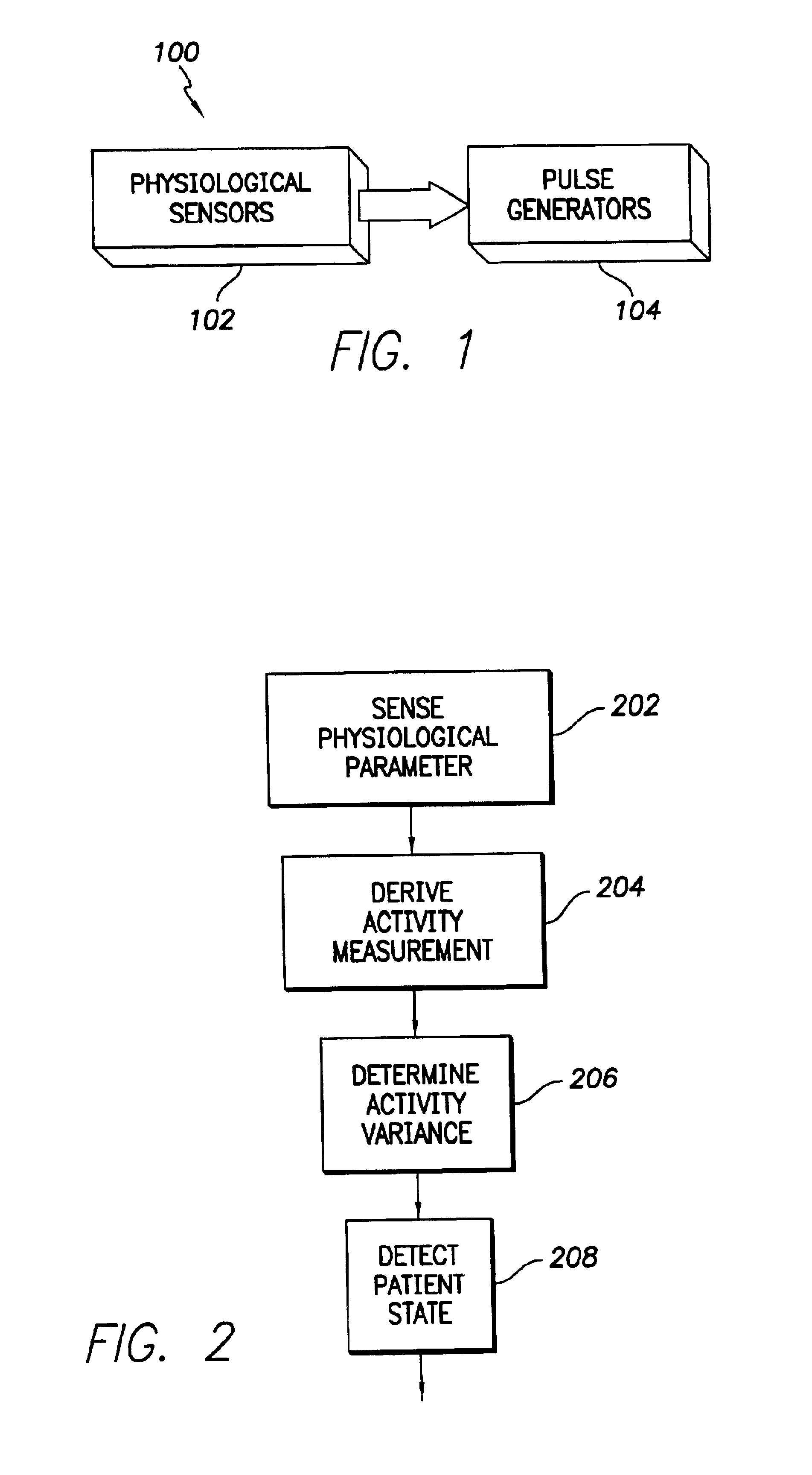
InactiveUS6975904B1Accurate lossAvoid captureHeart stimulatorsDiagnostic recording/measuringPatient statusPatient state
Optimization of evoked response detection in an automatic capture detection system employed by an implantable cardiac stimulation device is presented. Patient state information is used to determine the appropriate settings of variables that are associated with the evoked response signal detection algorithm. The variables used by the evoked response signal detection algorithm are first established for the patient in a variety of positions. During operation of the implantable cardiac stimulation device, the patient state is monitored and the variables used by the evoked response signal detection algorithm are adjusted accordingly.
Owner:PACESETTER INC
Method and apparatus for assessing left ventricular function and optimizing cardiac pacing intervals based on left ventricular wall motion
A system and method for monitoring left ventricular (LV) lateral wall motion and for optimizing cardiac pacing intervals based on left ventricular lateral wall motion is provided. The system includes an implantable or external cardiac stimulation device in association with a set of leads including a left ventricular epicardial or coronary sinus lead equipped with a motion sensor electromechanically coupled to the lateral wall of the left ventricle. The device receives and processes wall motion sensor signals to determine a signal characteristic indicative of systolic LV lateral wall motion or acceleration. An automatic pacing interval optimization method evaluates the LV lateral wall motion during varying pacing interval settings, including atrial-ventricular intervals and inter-ventricular intervals and selects the pacing interval setting(s) that correspond to LV lateral wall motion associated with improved cardiac synchrony and hemodynamic performance.
Owner:MEDTRONIC INC
Epicardial electrode
ActiveUS7085606B2Reduce riskEasy accessEpicardial electrodesSurgical instrument detailsCardiac musclePatch electrode
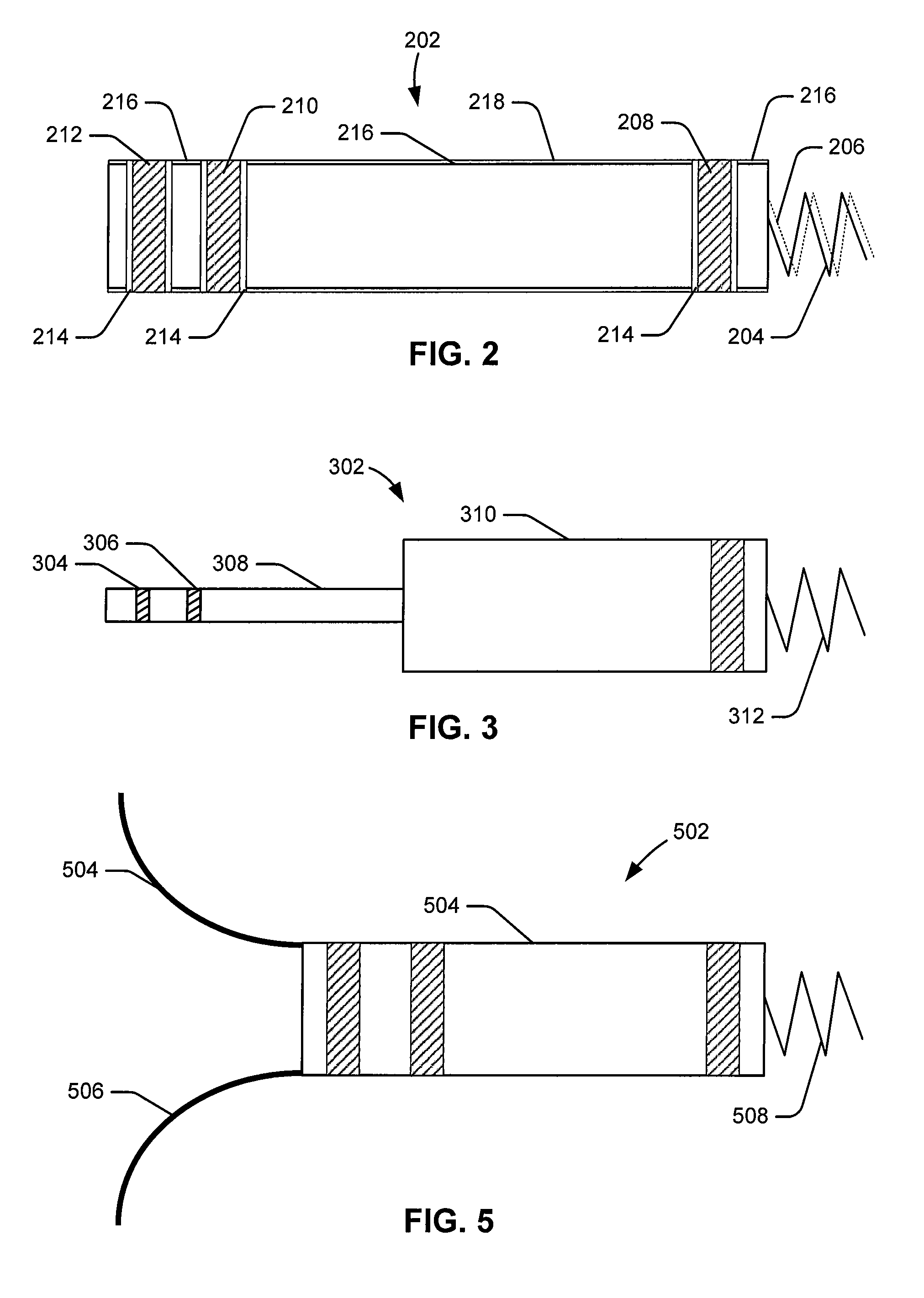
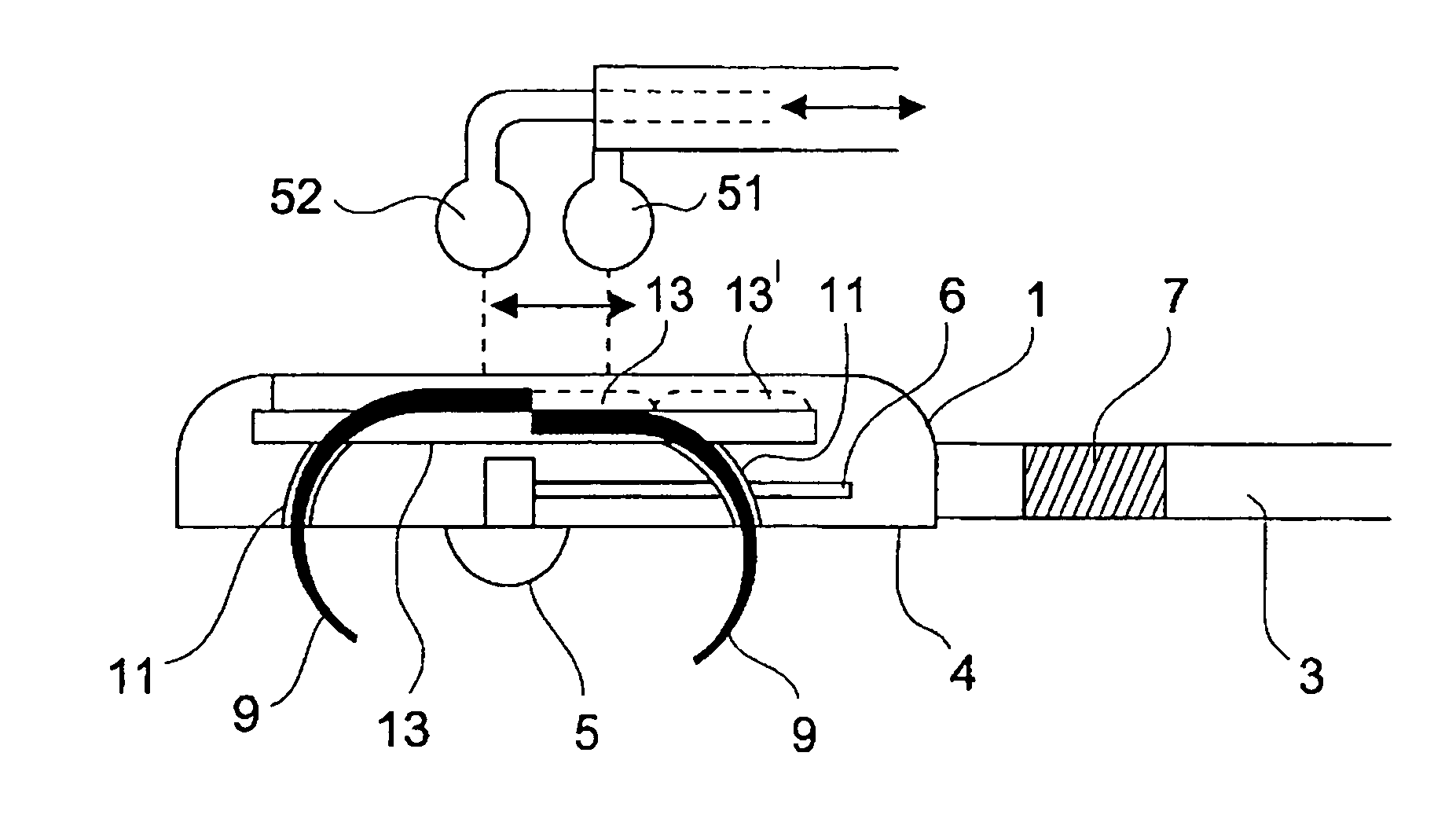
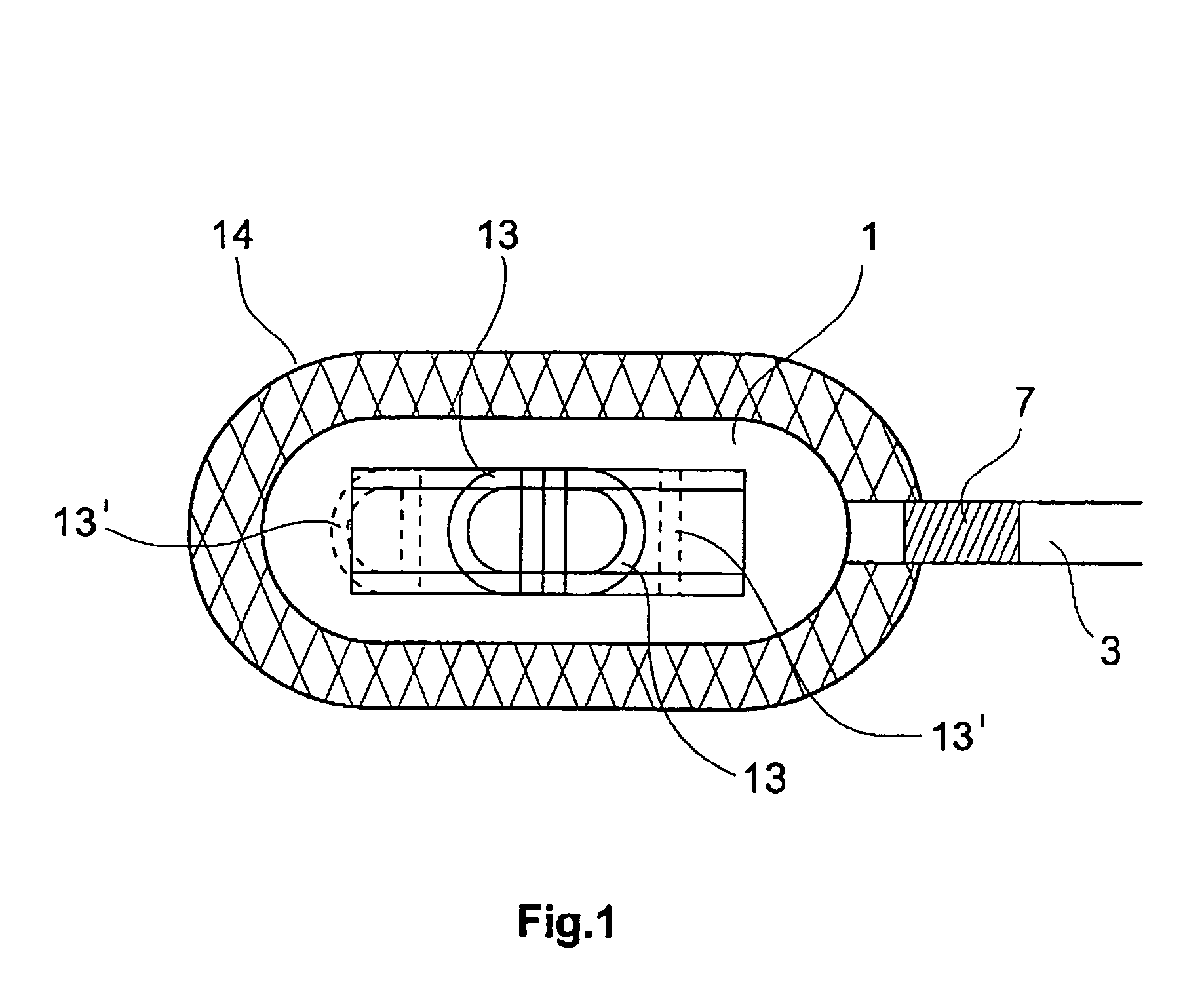
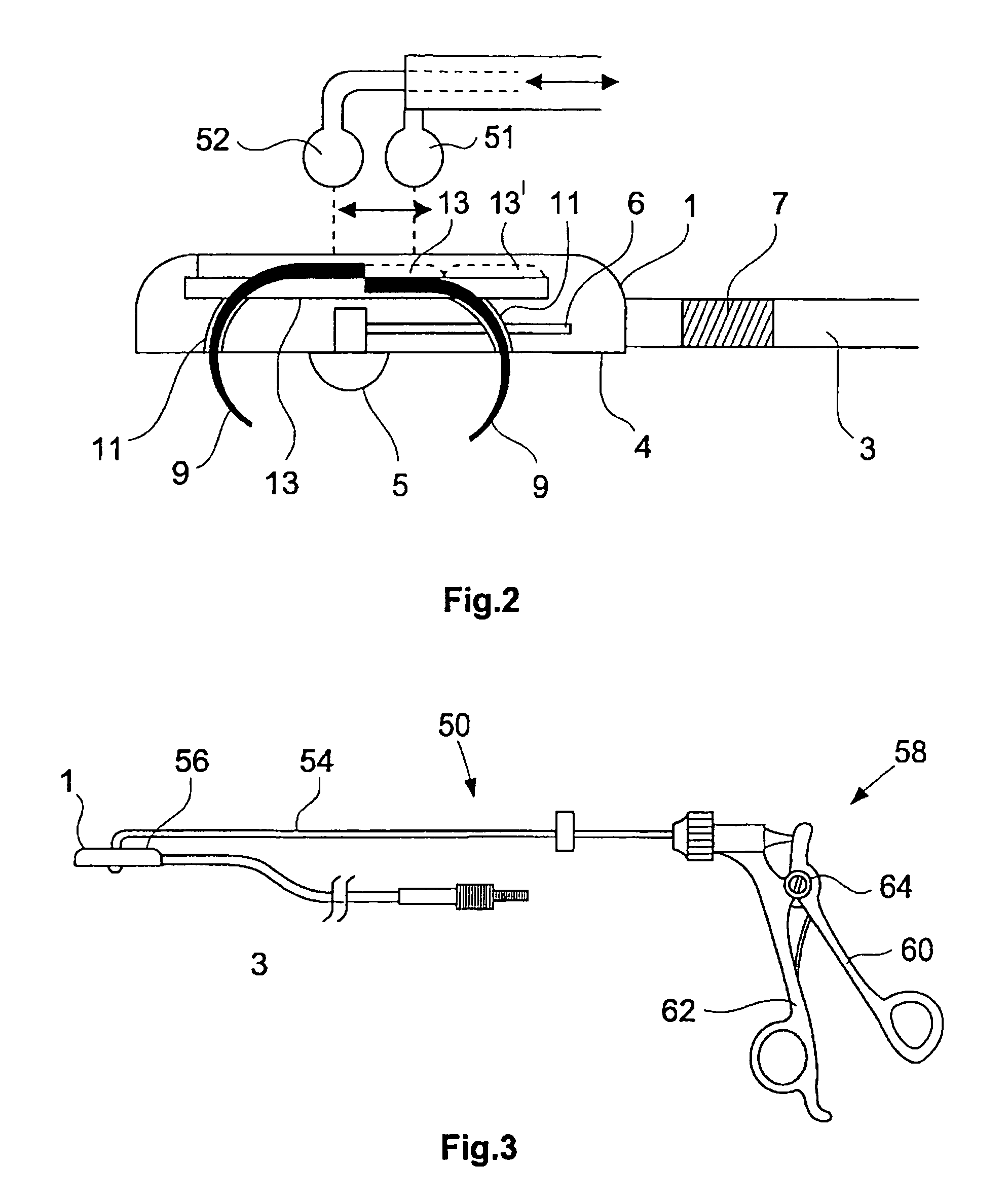
An epicardial electrode which is suitable, in particular, for use with a cardiac stimulation device, comprises an electrode body which has a stimulation surface adapted to bear against the cardiac tissue and to stimulate a part of the heart, that is to say a partial region of the heart, and at least one fixing element for fixing the stimulation surface to the cardiac tissue. The at least one fixing element is adapted for engagement into the cardiac tissue. The epicardial electrode can be secured to the outside and in particular to the outer skin of the cardiac muscle (epicardium) without being sewn to the cardiac muscle like a patch electrode. Only the fixing element has to be brought into engagement with the cardiac tissue.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Leadless intra-cardiac medical device with dual chamber sensing through electrical and/or mechanical sensing
ActiveUS8996109B2Small sizeConvenient to accommodateElectrocardiographyHeart stimulatorsCardiac activityRight atrium
A leadless intra-cardiac medical device senses cardiac activity from multiple chambers and applies cardiac stimulation to at least one cardiac chamber and / or generates a cardiac diagnostic indication. The leadless device may be implanted in a local cardiac chamber (e.g., the right ventricle) and detect near-field signals from that chamber as well as far-field signals from an adjacent chamber (e.g., the right atrium).
Owner:PACESETTER INC
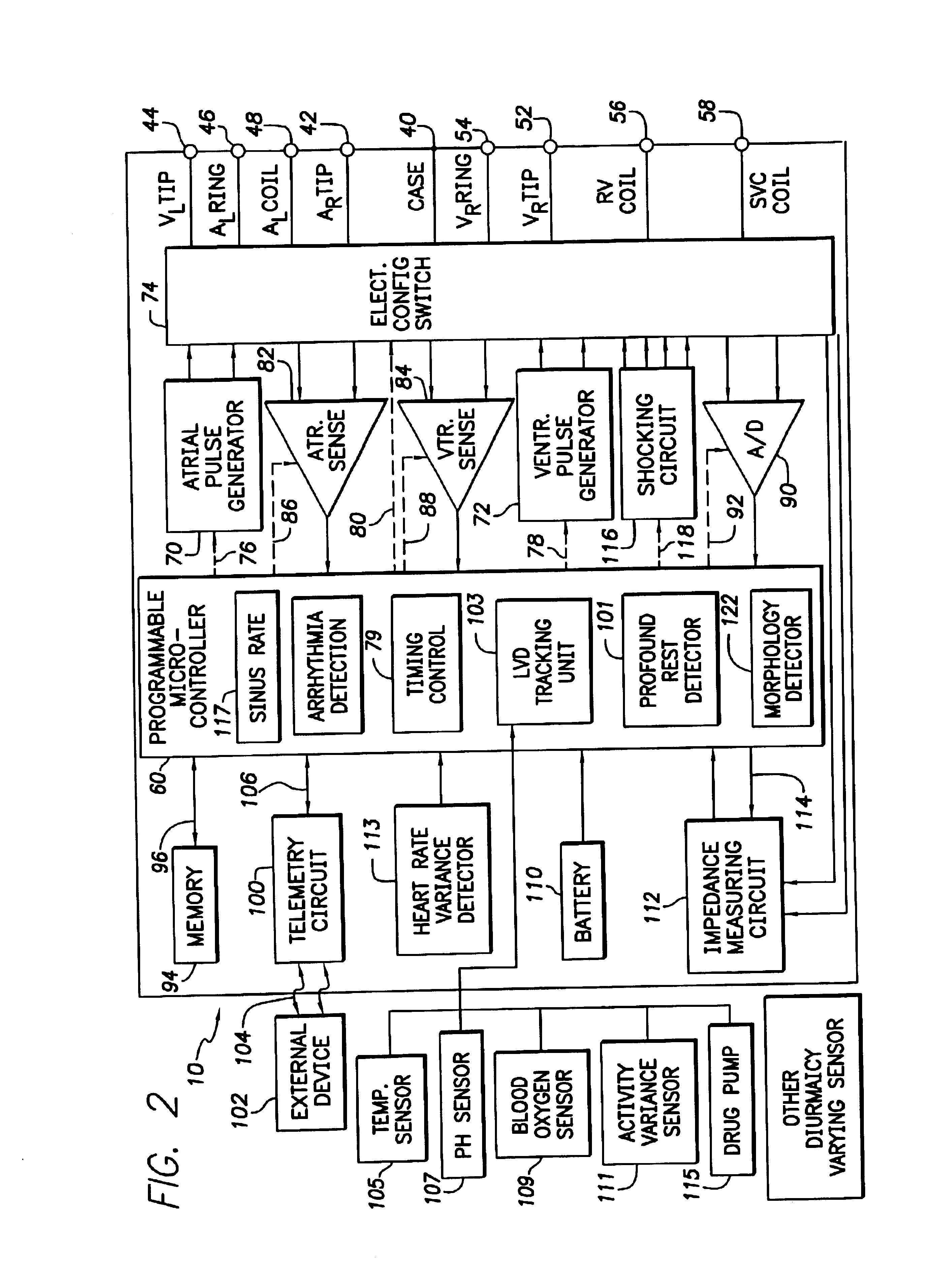
System and method for tracking progression of left ventricular dysfunction using implantable cardiac stimulation device
InactiveUS6922587B2Accurate and reliable assessmentAlter heart contractilityHeart stimulatorsPost extrasystolic potentiationCardiac pacemaker electrode
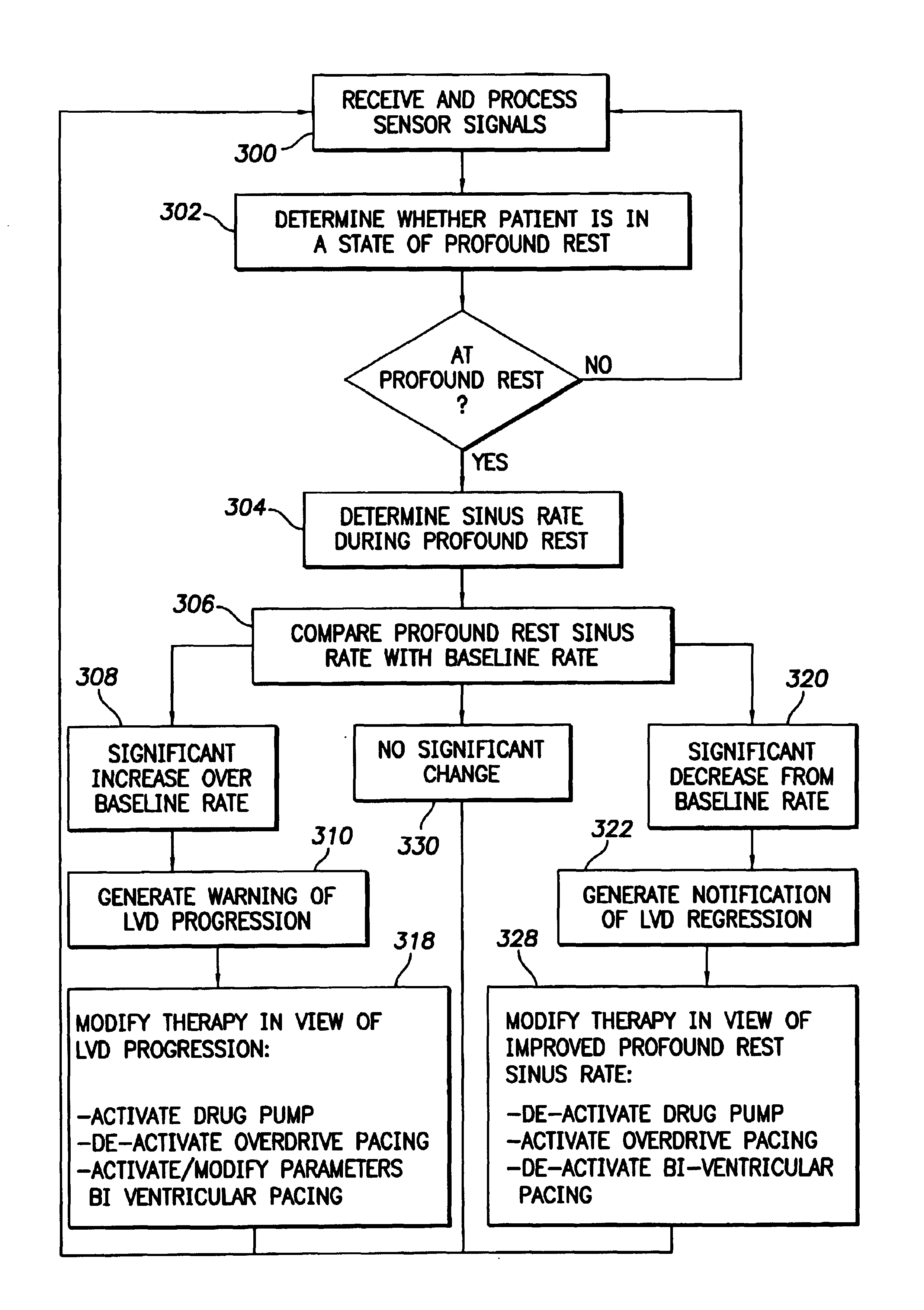
The progression or regression of left ventricular dysfunction (LVD) is automatically evaluated by a pacemaker or other implantable cardiac stimulation device by tracking changes in the resting sinus rate of the patient in which the device is implanted. The resting sinus rate is detected by first determining whether the patient is in a state of profound rest, such as sleep, then measuring the actual sinus rate during profound rest. Profound rest may be detected by using an activity variance sensor. An increase in the profound rest sinus rate over a period of several months indicates progression of LVD; whereas a decrease indicates regression. Appropriate LVD diagnostic information is recorded for subsequent review by a physician. Based on the progression or regression of LVD, the physician may then modify LVD drug therapy administered to the patient or may adjust control parameters of the pacemaker, such as overdrive pacing control parameters or control parameters affecting heart contractility via post-extrasystolic potentiation. If a drug pump is implanted within the patient for automatically delivering LVD drug therapy, the pacemaker controls the drug pump in view of any detected progression or regression of LVD. The technique may also be used to verify the efficacy of LVD drug therapy administered to the patient, whether delivered via an implanted drug pump or otherwise. Processing may be primarily performed within the implanted device itself or with an external programmer in communication with the implanted device. Activity state-based LVD tracking techniques are also set forth.
Owner:PACESETTER INC
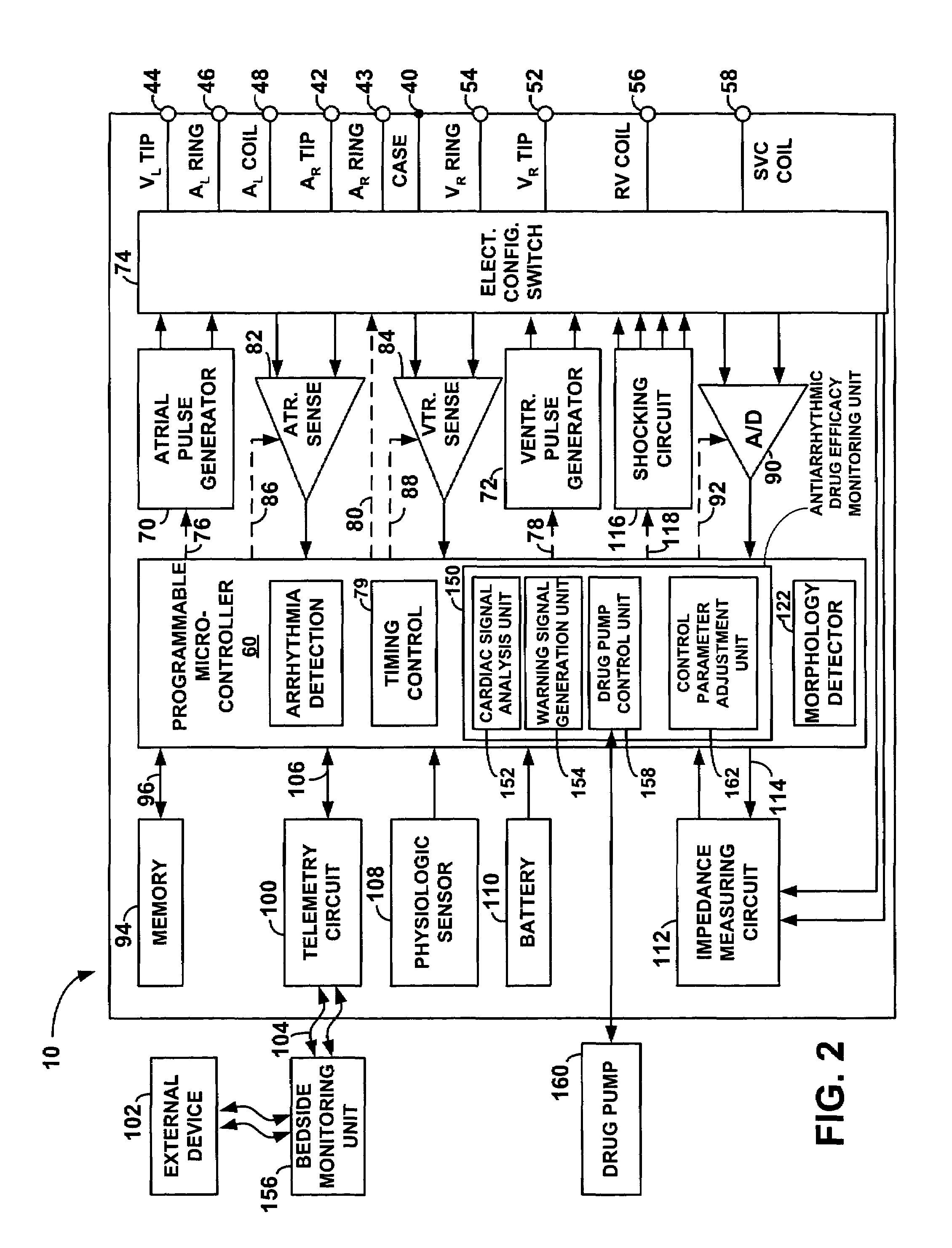
Method and apparatus for monitoring drug effects on cardiac electrical signals using an implantable cardiac stimulation device
InactiveUS7142911B2Increase aggressivenessIncrease dosePhysical therapies and activitiesDrug and medicationsEcg signalCardiac pacemaker electrode
An implantable cardiac stimulation device, such as a pacemaker or Implantable Cardioverter Defibrillator, is configured to automatically monitor the effects of antiarrhythmic drugs on cardiac electrical signals within a patient to verify the efficacy of the drugs taken. In one example, an analysis of patient cardiac electrical signals is performed by comparing the cardiac electrical signals with values representative of the effects of different classes of antiarrhythmic drugs. If the implantable device determines that the prescribed antiarrhythmic drugs have not been effective, a warning signal is generated. The warning signal is conveyed directly to the patient via a bedside monitor and to the patient's physician via remote connection to an external programmer device so that both are notified of the drug efficacy problems. Additionally, the implantable device may be configured to automatically adjust pacing and defibrillation control parameters in an attempt to compensate for any lack of efficacy in the drugs. For example, the aggressiveness of overdrive pacing may be increased. Alternatively, a drug pump is controlled to adjust the dosage of antiarrhythmic drugs if an initial dosage is found to be ineffective.
Owner:PACESETTER INC
Medical diagnostic and communication system
InactiveUS20070032832A1Expand coverageImprove performanceSpatial transmit diversityModulated-carrier systemsHeart pacemakersEmergency rooms
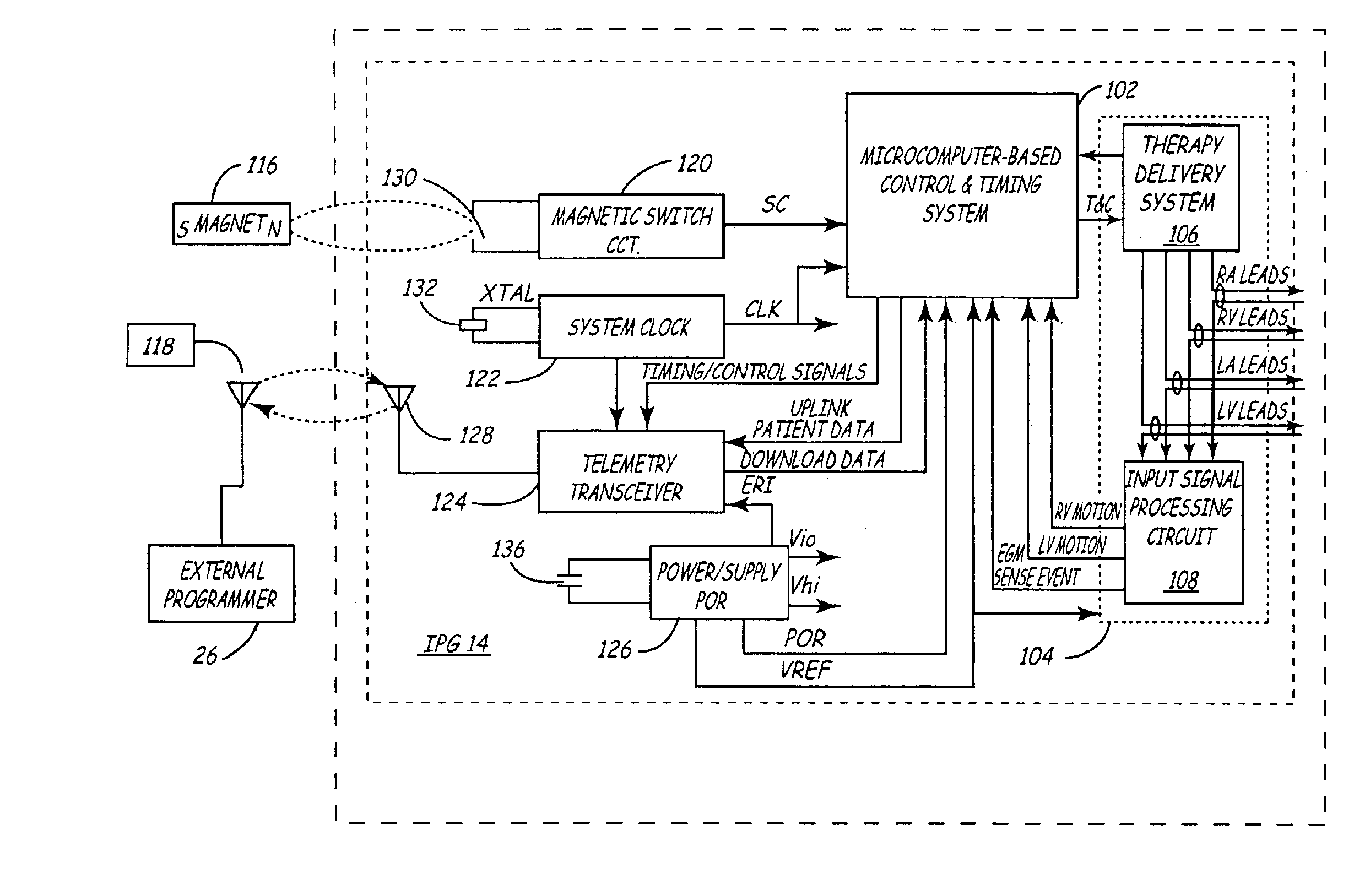
Cardiac stimulation device and wireless communication system, without magnetic detection or magnetic control of the heart pacemaker parameters, having leads for carrying stimulating pulses to and or from one or more electrodes located in a heart and a pulse generator configured to generate stimulation pulses. In certain embodiments and environments the heart pacemaker could operate in an emergency room, even during Magnetic Resonance Imaging (MRI) diagnostic studies. A processor for connection of the stimulating pulses to and / or from one or more spread spectrum transmitter-receiver (T / R) circuits and / or from a signal processing network for receiving said stimulation pulses and for providing cross-correlated in-phase and quadrature-phase baseband signals. One or more modulators and demodulators for transmission and / or reception of one or more spread spectrum and / or cross-correlated signals.
Owner:FEHER KAMILO
Cardiac stimulation system
ActiveUS20140039591A1Reduced effectivenessReduce injuriesEpicardial electrodesDiagnostic recording/measuringHeart chamberEngineering
Some embodiments of pacing systems employ wireless electrode assemblies to provide pacing therapy. The wireless electrode assemblies may wirelessly receive energy via an inductive coupling so as to provide electrical stimulation to the surrounding heart tissue. In certain embodiments, the wireless electrode assembly may include one or more biased tines that shift from a first position to a second position to secure the wireless electrode assembly into the inner wall of the heart chamber.
Owner:BOSTON SCI SCIMED INC
Mechanical ventricular pacing capture detection for a post extrasystolic potentiation (PESP) pacing therapy using at least one lead-based accelerometer
InactiveUS20080234771A1Improve cardiac perfusionDecrease in cardiac performanceCatheterHeart stimulatorsPost extrasystolic potentiationAccelerometer
A system and method for monitoring at least one chamber of a heart (e.g., a left ventricular chamber) during delivery of extrasystolic stimulation to determine if the desired extra-systole (i.e., ventricular mechanical capture following refractory period expiration) occurs. The system includes an implantable or external cardiac stimulation device in association with a set of leads such as epicardial, endocardial, and / or coronary sinus leads equipped with motion sensor(s). The device receives and processes acceleration sensor signals to determine a signal characteristic indicative of chamber capture resulting from one or more pacing stimulus delivered closely following expiration of the refractory period. A threshold optimization method optionally evaluates capture and at least one of: runs an iterative routine to establish or re-establish chamber capture for the PESP therapy, sets a logical flag relating to chamber capture status and stores parameter(s) relating to successful chamber capture for one or more subsequent cardiac cycles.
Owner:MEDTRONIC INC
Implantable cardiac stimulation device and method that discriminates between and treats atrial tachycardia and atrial fibrillation
An implantable cardiac stimulation device discriminates and treats accelerated atrial arrhythmias of a patient's heart. The device includes a sensing circuit that senses cardiac activity of one of the patient's atria to provide an atrial activity signal, a detector that detects an accelerated atrial arrhythmia of the patient's heart, and a classifying circuit that measures relative correspondence between successive P waves of the atrial activity signal to classify the detected accelerated atrial arrhythmia as either atrial tachycardia or atrial fibrillation. A therapy circuit provides anti-tachycardia pacing therapy responsive to a classified atrial tachycardia and defibrillation therapy responsive to a classified atrial fibrillation.
Owner:PACESETTER INC
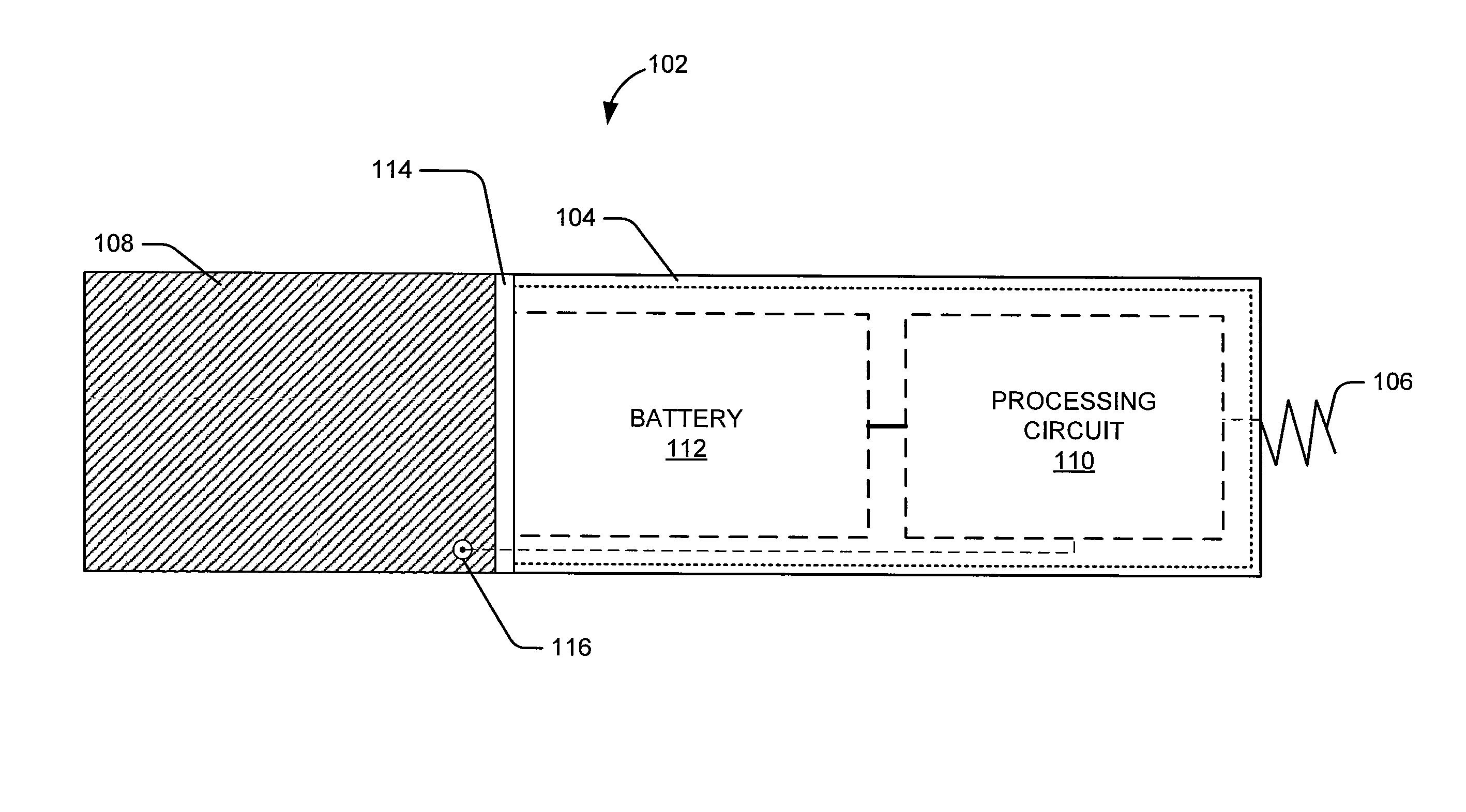
Reconfigurable implantable cardiac monitoring and therapy delivery device
Methods and systems for implantable cardiac monitors reconfigurable to cardiac therapy devices. A device includes a housing with electrodes configured for cardiac activity sensing when the device is operated in monitoring mode, and sensing and energy delivery when operated in an energy delivery mode. A header may be configured to receive a cardiac lead, and may be associated with a switch to switch the device between monitoring and therapy modes in response to connecting one or more leads to the header. The device may include a transceiver that transmits the contents of the memory to a patient-external device. A method involves providing an implantable cardiac device configured to operate in a first mode as a loop recorder for monitoring cardiac activity and storing selected cardiac events, and operating in the second mode to monitor cardiac activity and provide cardiac stimulation therapy when the second mode is enabled.
Owner:CARDIAC PACEMAKERS INC
Mechanical Ventricular Pacing Non-Capture Detection for a Refractory Period Stimulation (RPS) Pacing Therapy Using at Least One Lead-Based Accelerometer
InactiveUS20080269825A1Increase contractilityIncrease perfusionElectrotherapyDiagnostic recording/measuringAccelerometerLeft ventricular size
A system and method for monitoring at least one chamber of a heart (e.g., a left ventricular chamber) during delivery of a refractory period stimulation (RPS) therapy to determine if the desired non-capture (i.e., lack of ventricular mechanical capture due to refractory period stimulation) occurs. The system includes an implantable or external cardiac stimulation device in association with a set of leads such as epicardial, endocardial, and / or coronary sinus leads equipped with motion sensor(s). The device receives and processes acceleration sensor signals to determine a signal characteristic indicative of chamber capture due to pacing stimulus delivery, non-capture due to RPS therapy delivery, and / or contractile status based on the qualities of evoked response to pacing stimulation.
Owner:MEDTRONIC INC
Leadless cardiac stimulation device employing distributed logic
ActiveUS8818504B2Epicardial electrodesTransvascular endocardial electrodesCardiac activityCardiac stimulation
Systems and methods involve an intrathoracic cardiac stimulation device operable to provide autonomous cardiac sensing and energy delivery. The cardiac stimulation device includes a housing configured for intrathoracic placement relative to a patient's heart. A fixation arrangement of the housing is configured to affix the housing at an implant location within cardiac tissue or cardiac vasculature. An electrode arrangement supported by the housing is configured to sense cardiac activity and deliver stimulation energy to the cardiac tissue or cardiac vasculature. Energy delivery circuitry in the housing is coupled to the electrode arrangement. Detection circuitry is provided in the housing and coupled to the electrode arrangement. Communications circuitry may optionally be supported by the housing. A controller in the housing coordinates delivery of energy to the cardiac tissue or cardiac vasculature in accordance with an energy delivery protocol appropriate for the implant location.
Owner:CARDIAC PACEMAKERS INC
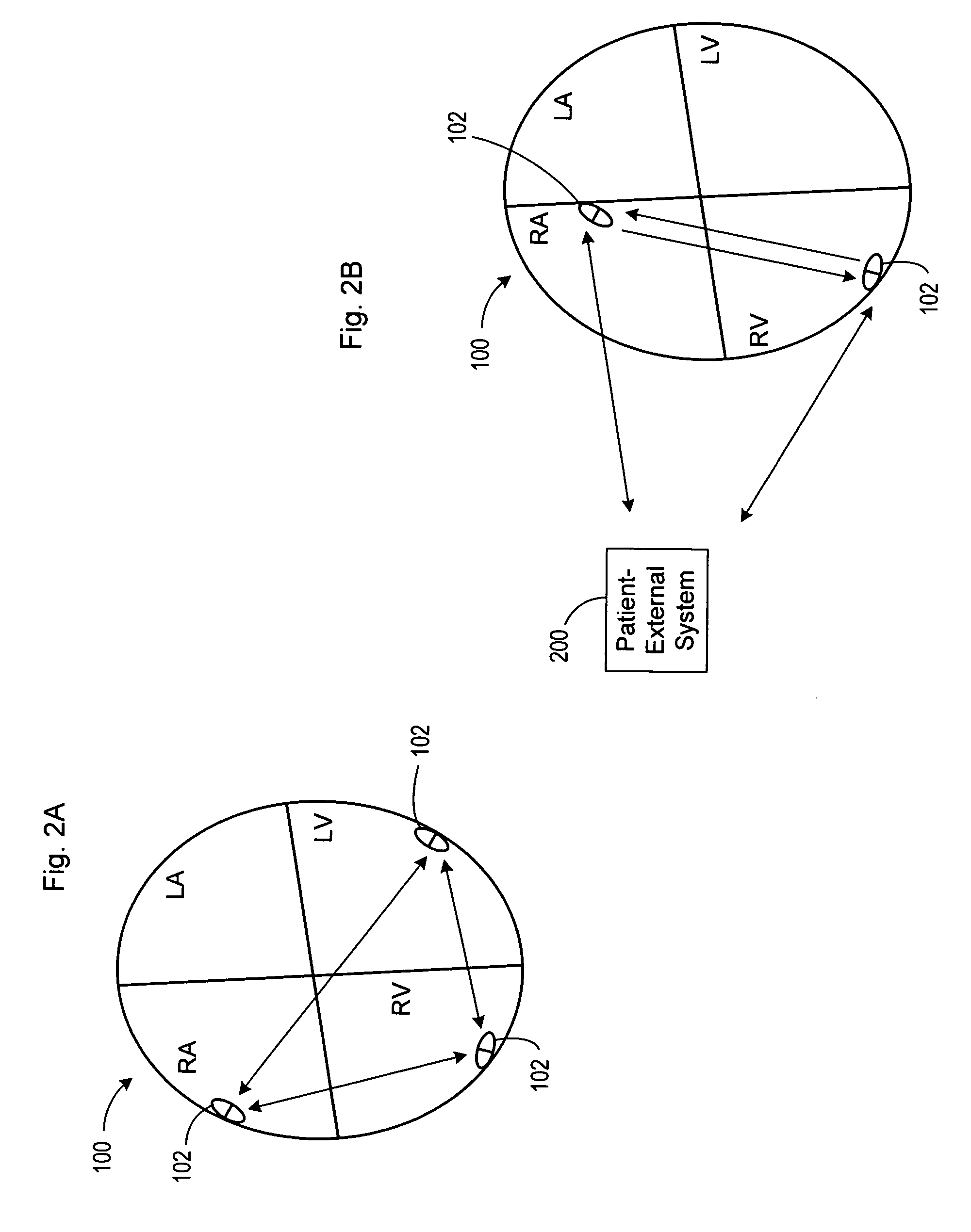
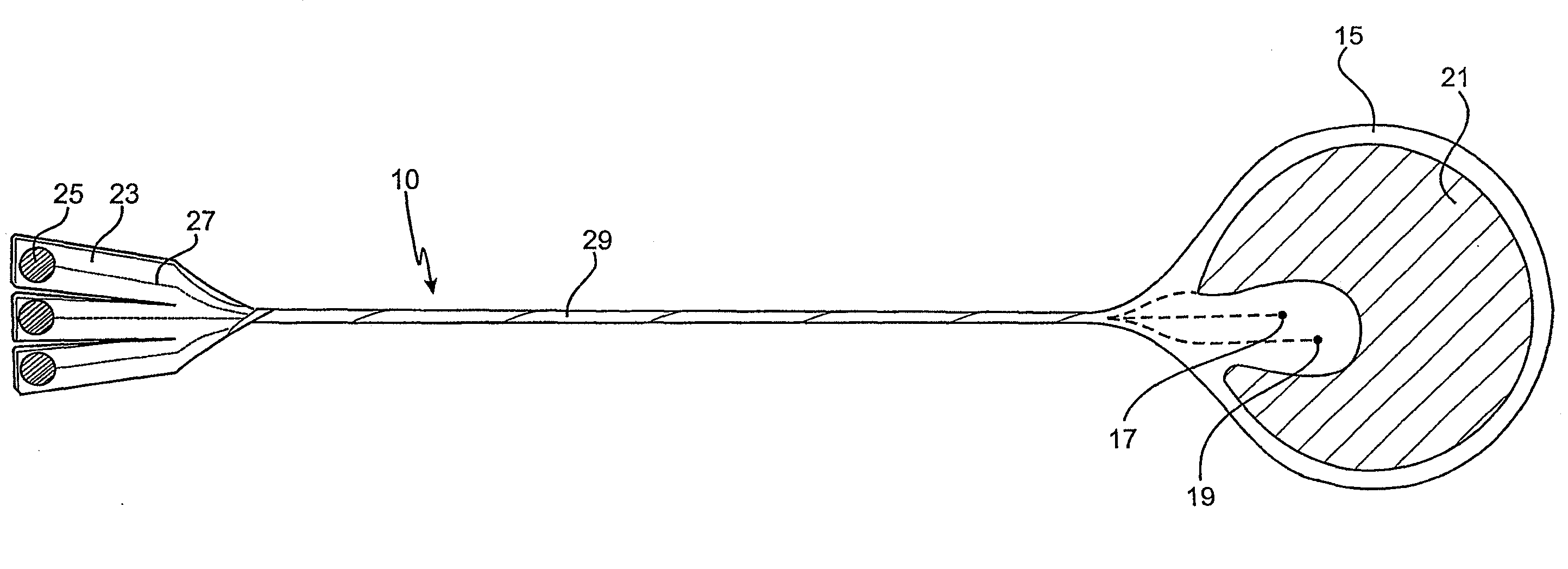
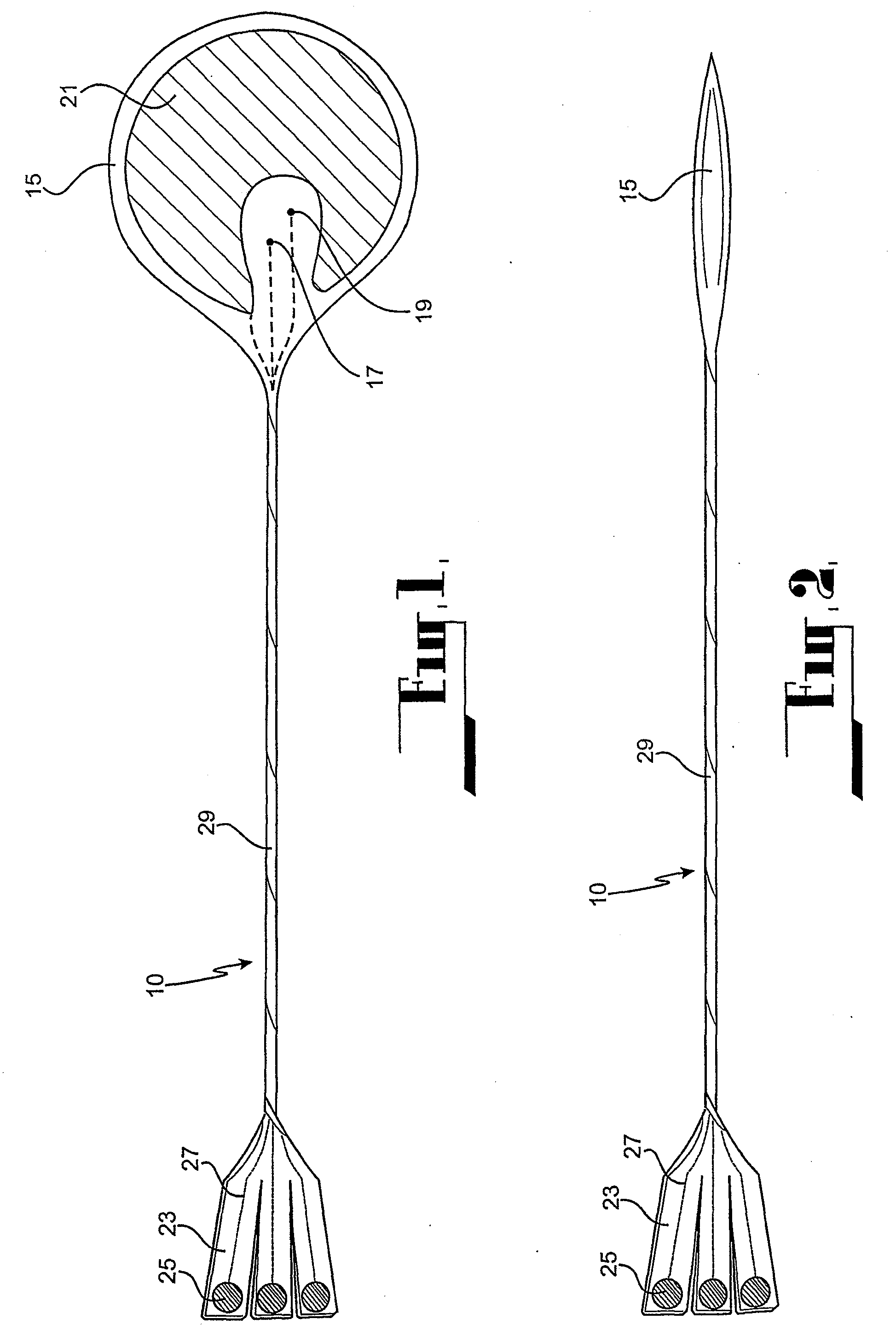
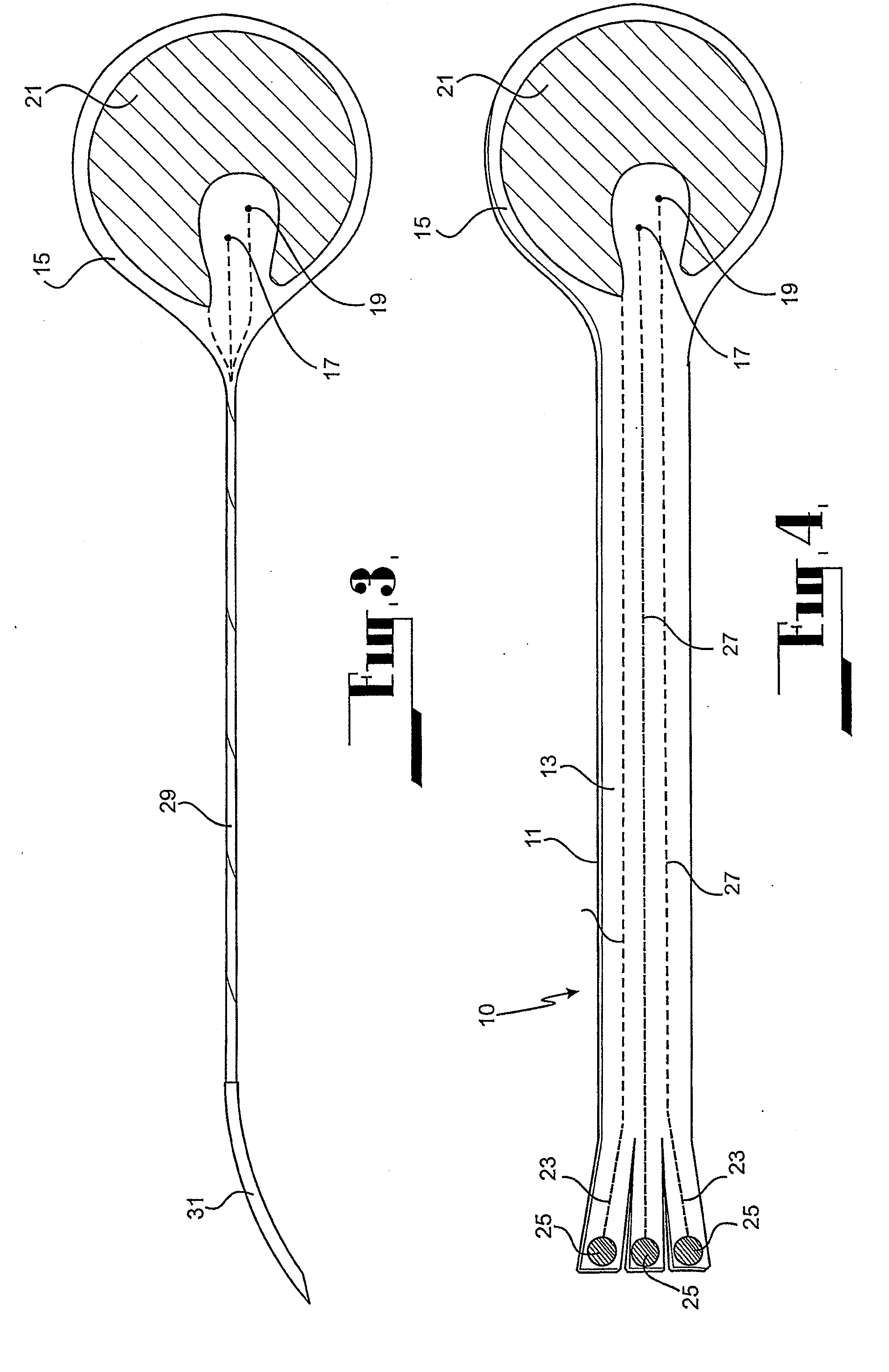
Cardiac stimulation apparatus
InactiveUS20080243217A1Eliminate needEpicardial electrodesDiagnostic recording/measuringNon traumaticBiomedical engineering
Cardiac stimulation apparatus (10) such as a lead comprising one or more electrodes (17, 19 and 21) for electrical contact with heart or other tissue. An adhesive substance is provided for adhesively attaching the electrodes in position in relation to the heart or other tissue to provide a non-traumatic fixing procedure. The electrodes (17, 19, 21) are positioned on a support (15) to which the adhesive substance is applied attached.
Owner:WILDON MICHAEL PETER
Evoked response variability as an indicator of autonomic tone and surrogate for patient condition
Modern implantable cardiac stimulation devices include processing and data storage capabilities that may be exploited to track myocardial condition and autonomic tone. Implantable devices have a capability to measure and store electrogram information over a period of time in a relatively large capacity memory, with advances in technology allowing increases in memory size. The evoked response varies in amplitude and morphology with changes in autonomic tone, ventricular filling, paced rate, and other parameters. The implantable cardiac device can be configured to sense and accurately quantify the evoked response, derive parameters from the quantified evoked response, store the parameters over long time periods, and derive variability statistics from the parameters to assist in tracking the patient's condition over time, and guiding the patient's therapy.
Owner:PACESETTER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com