Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Cardiac implanted" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
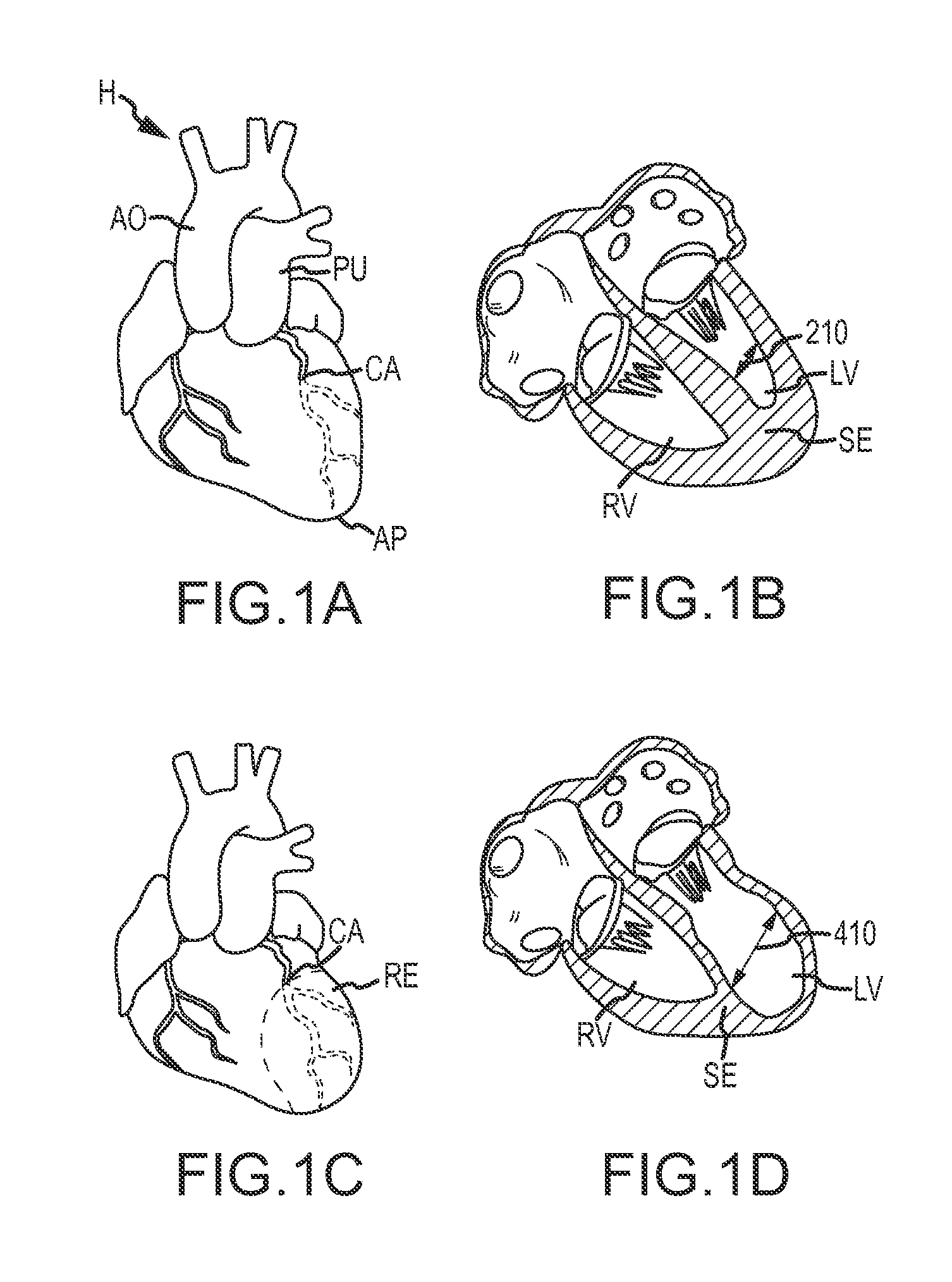
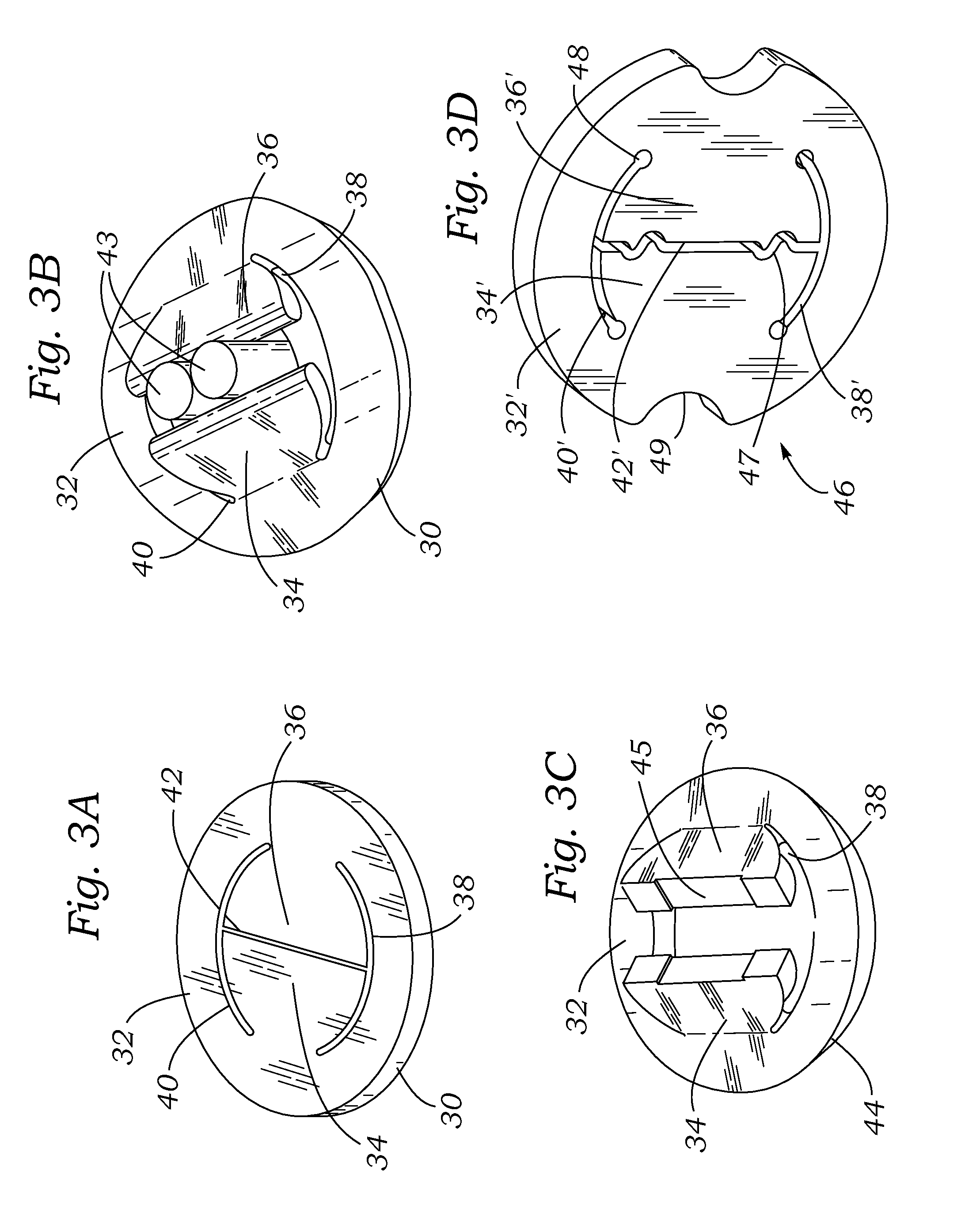
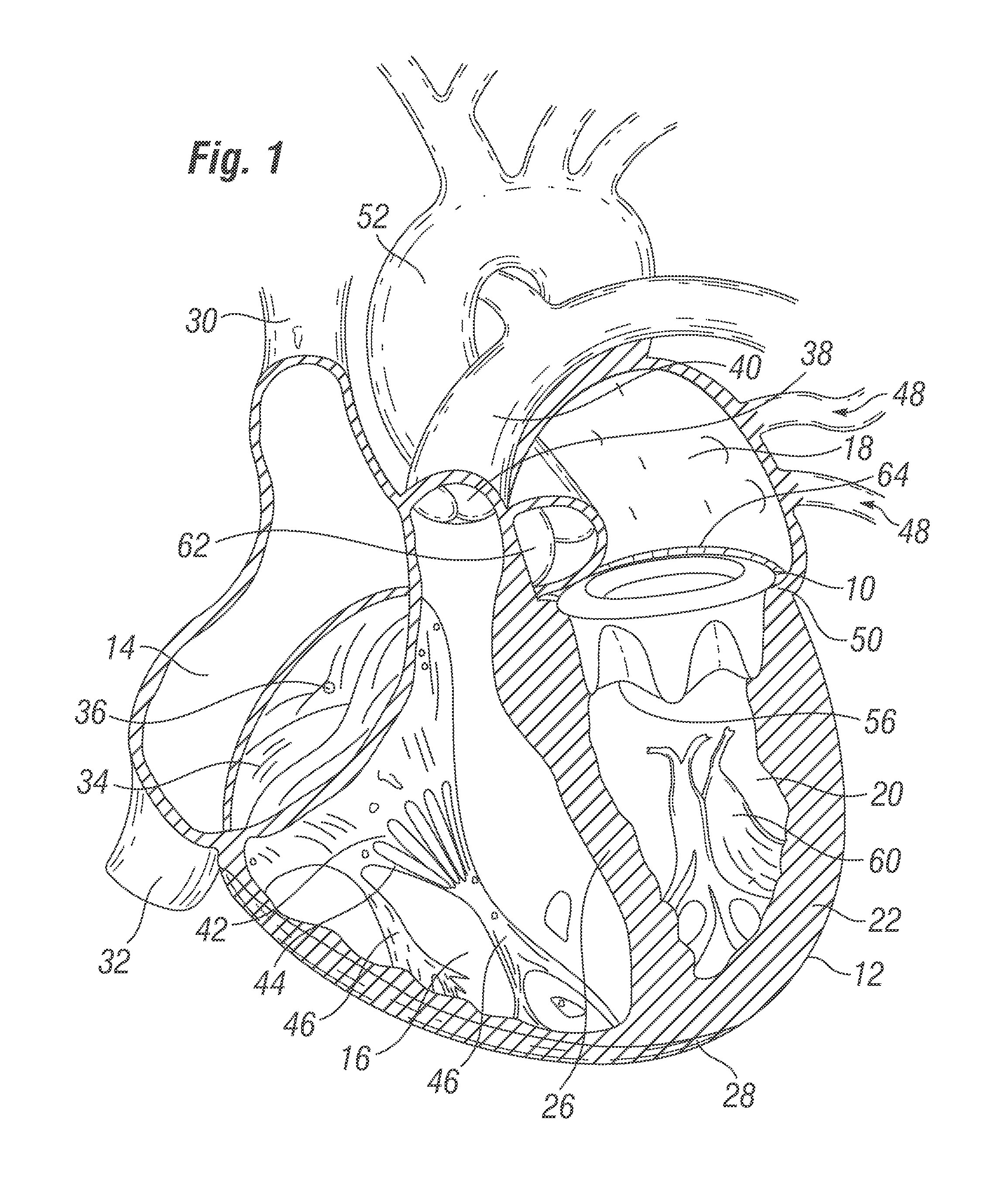
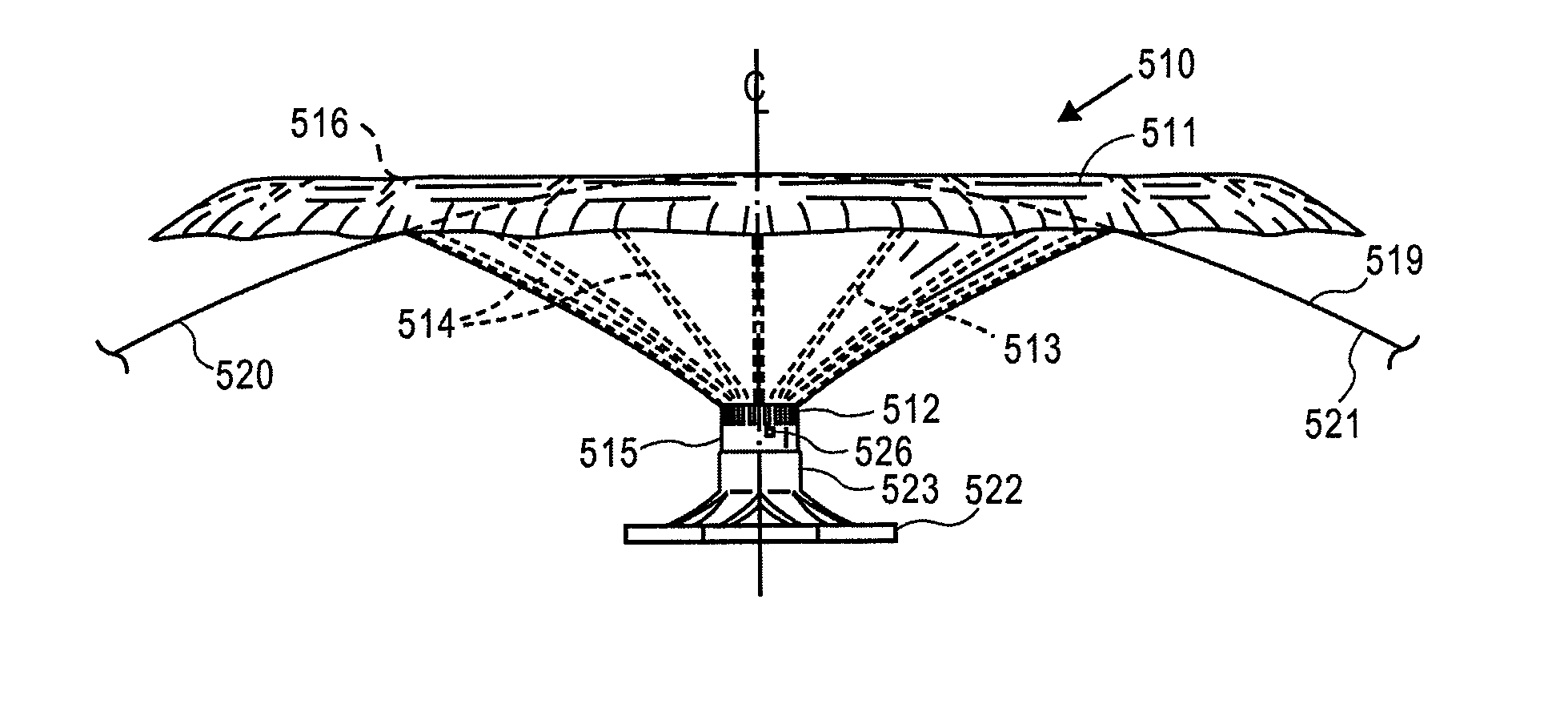
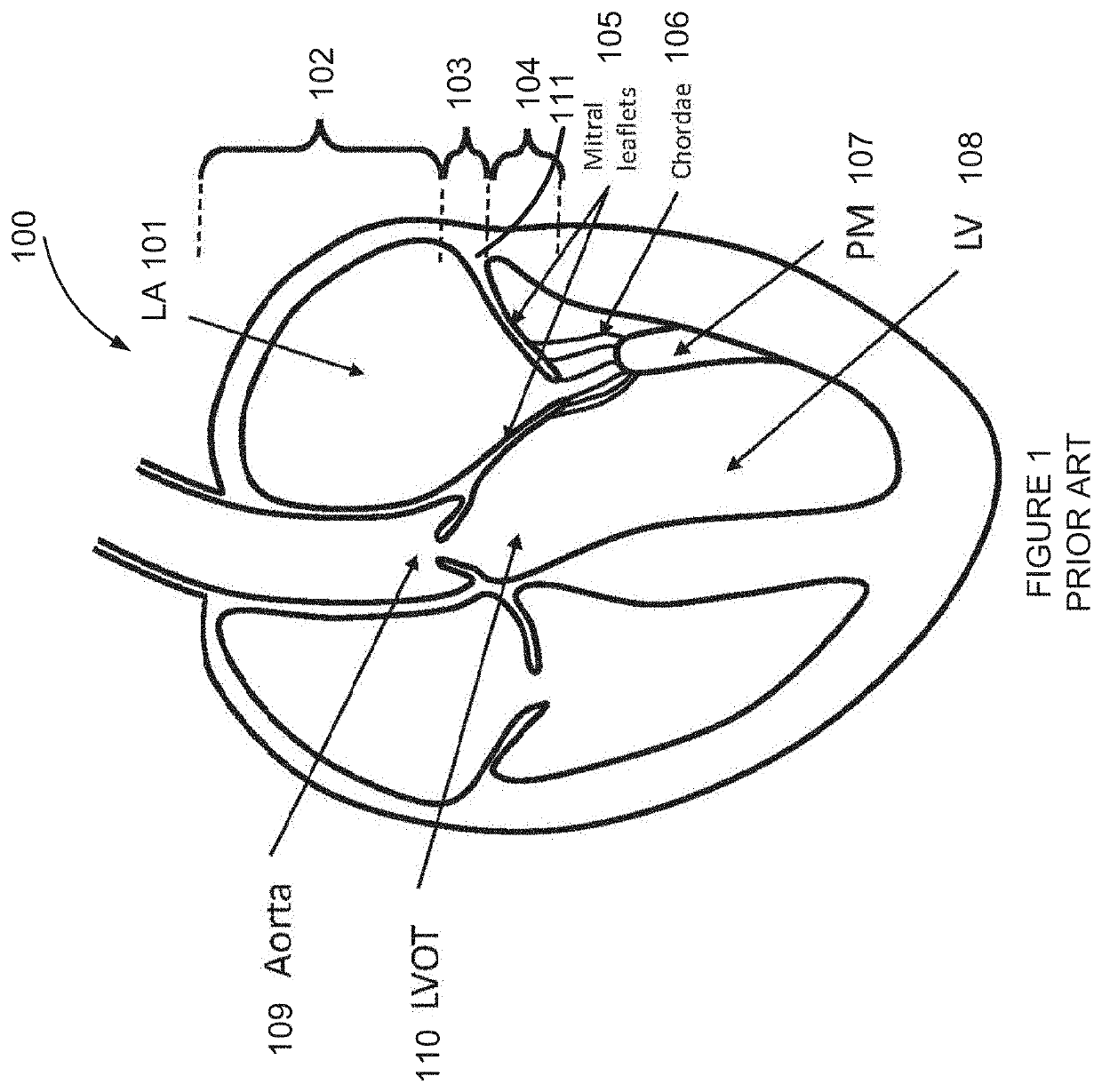
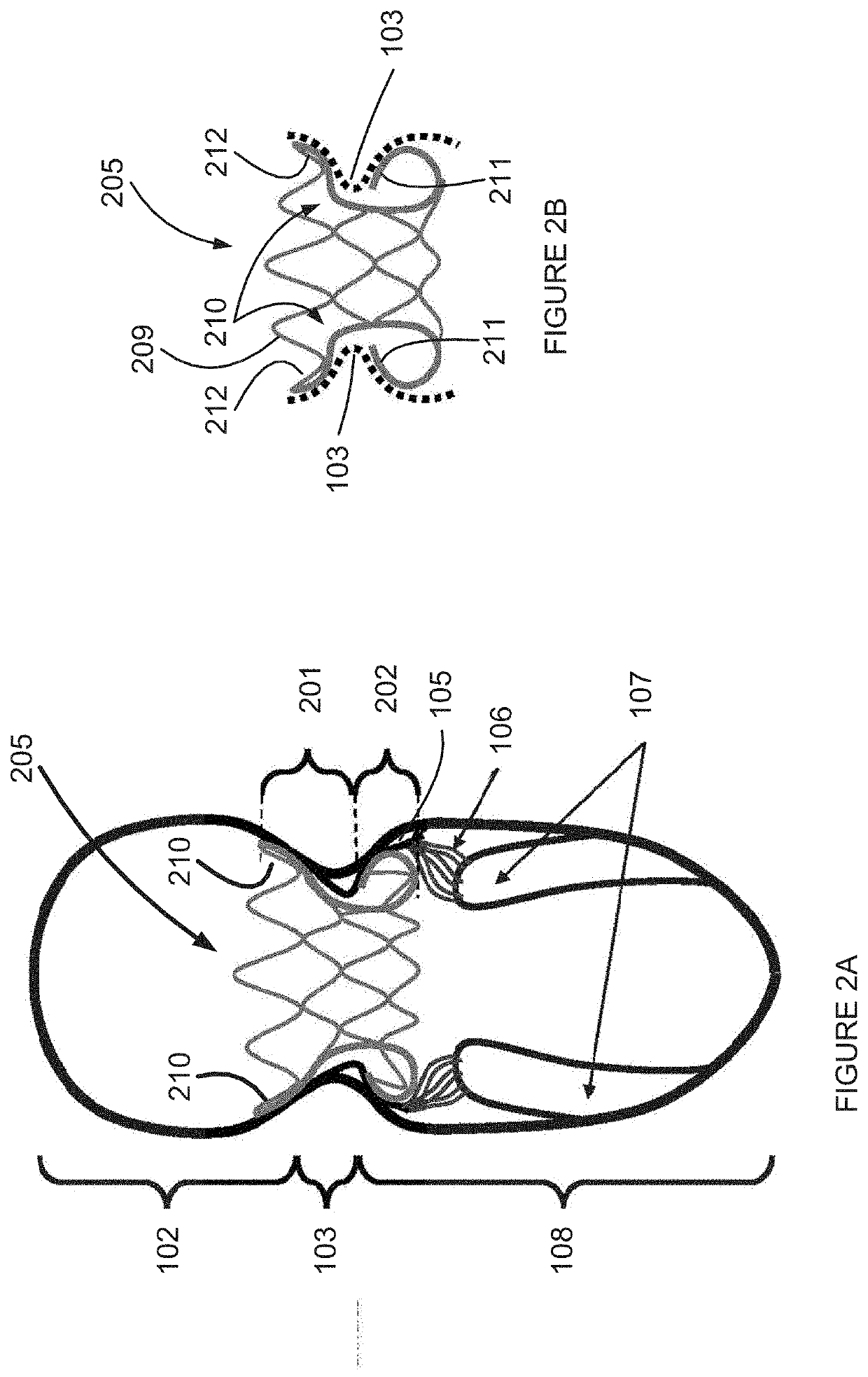
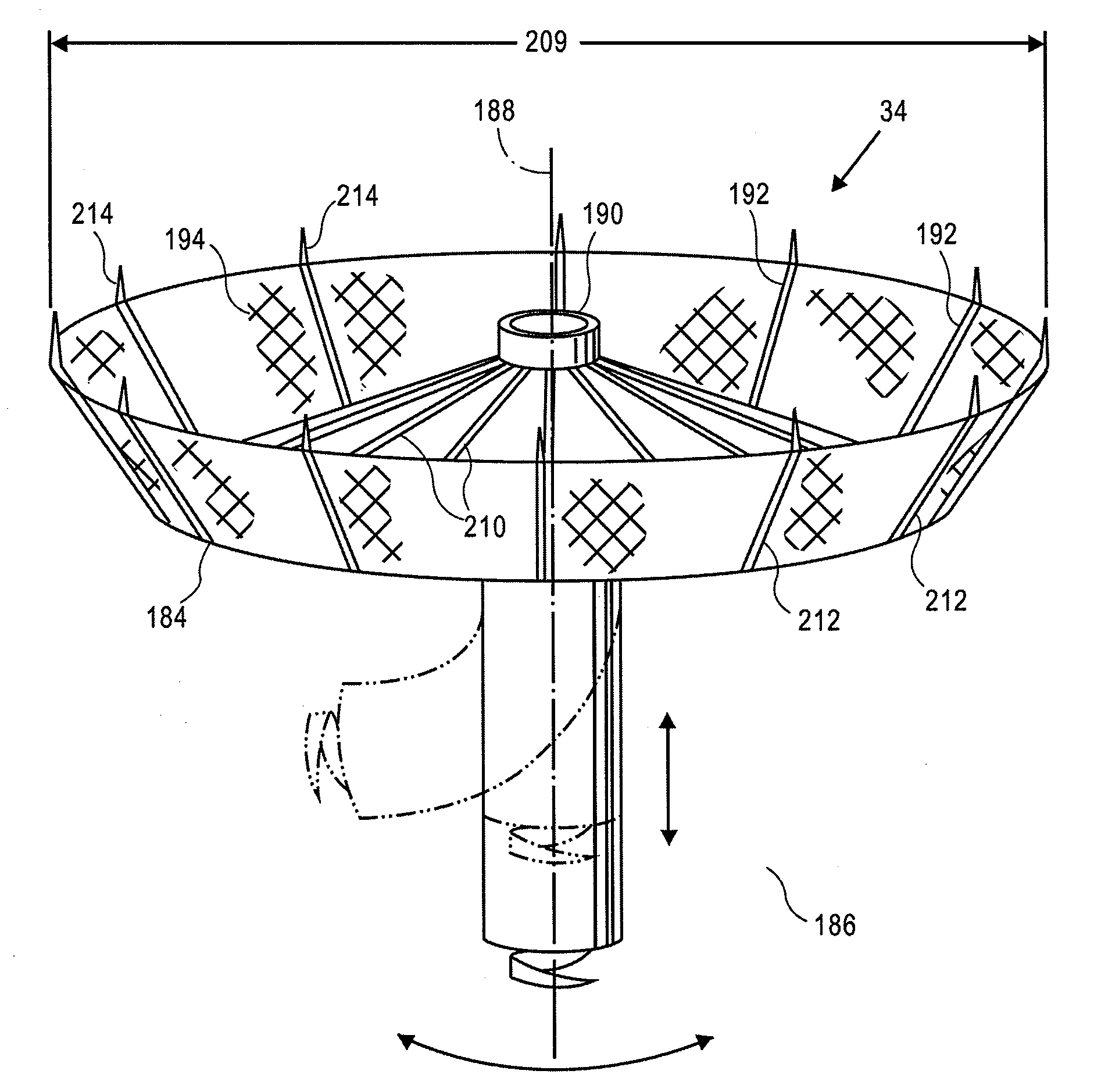
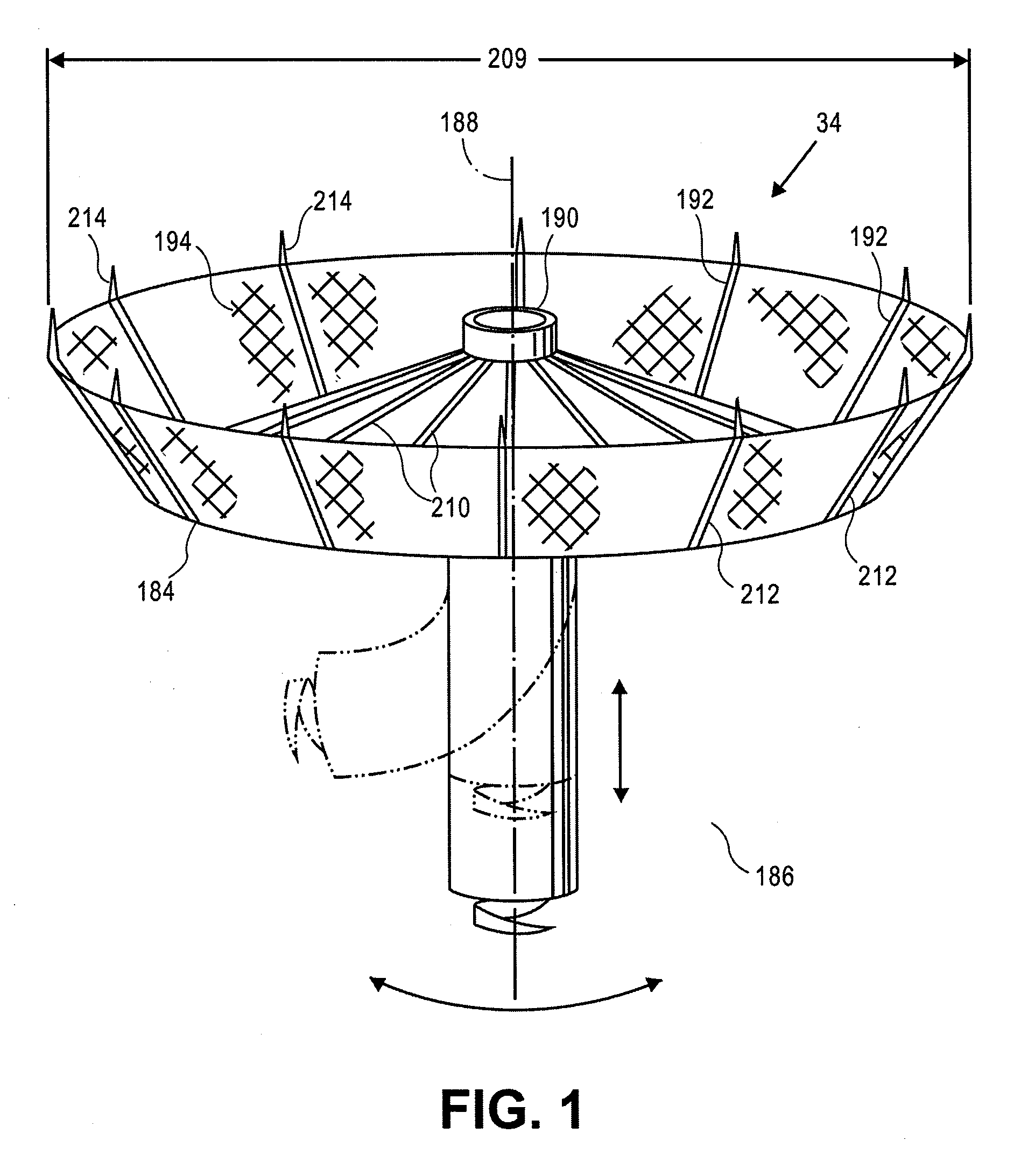
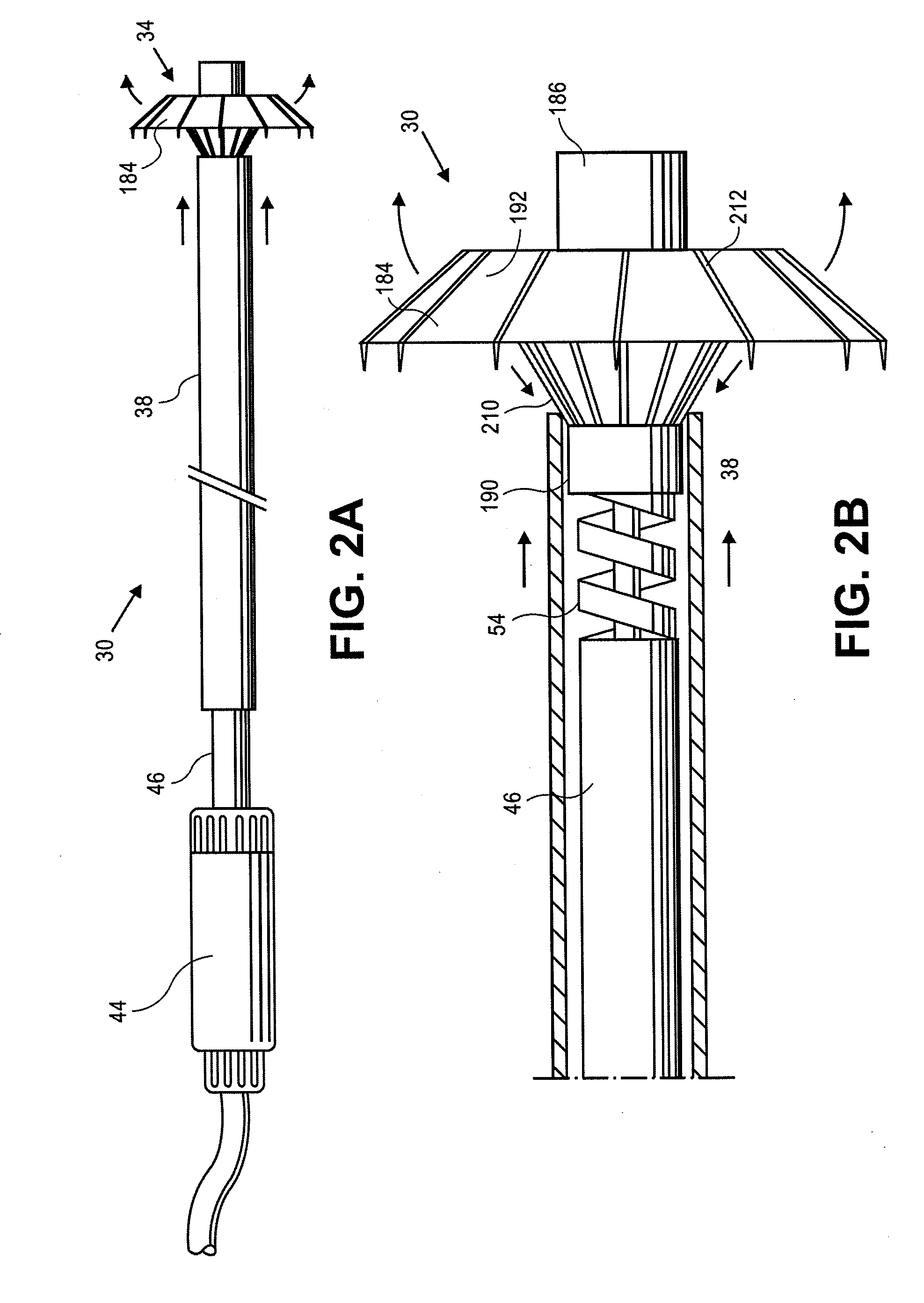
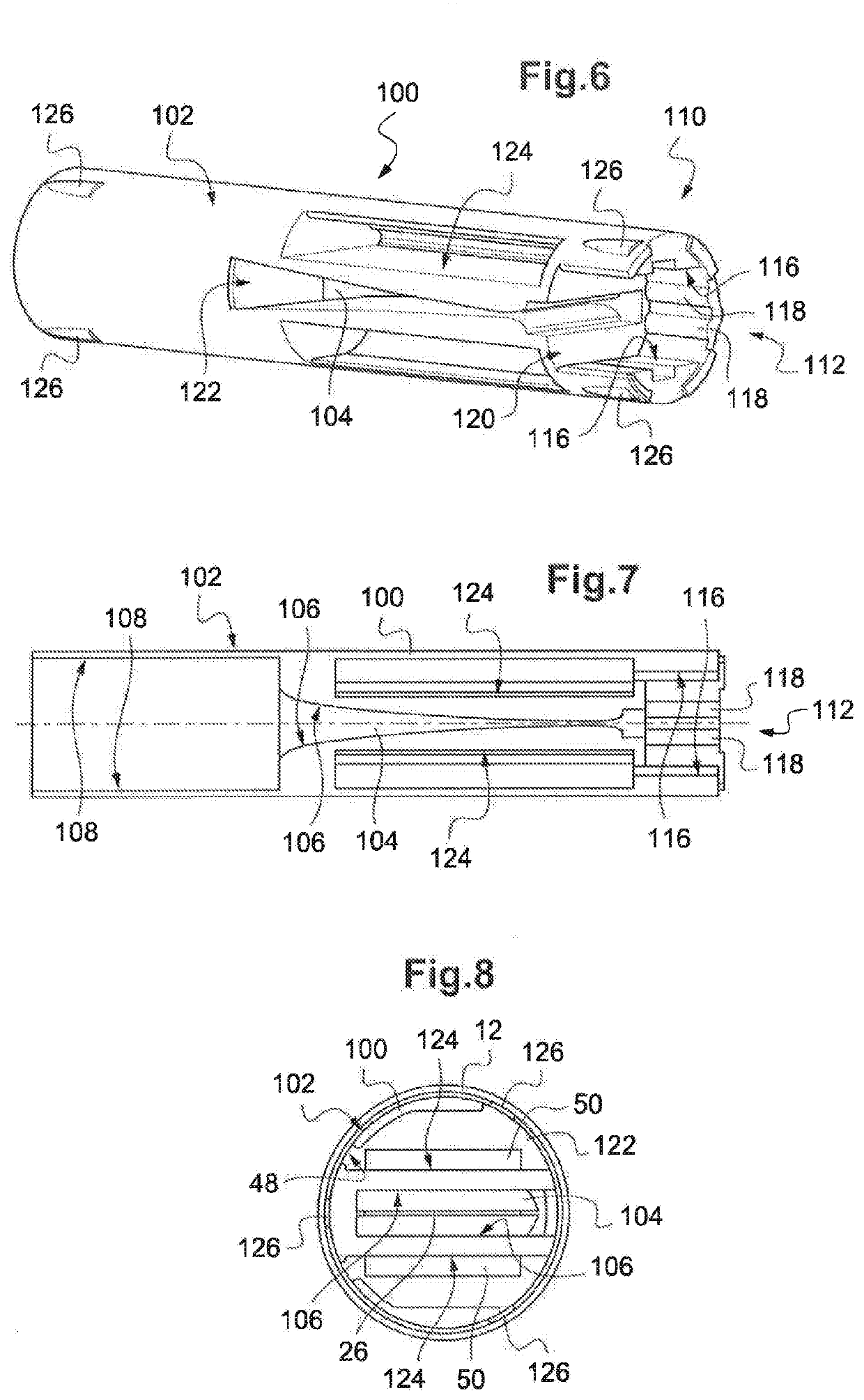
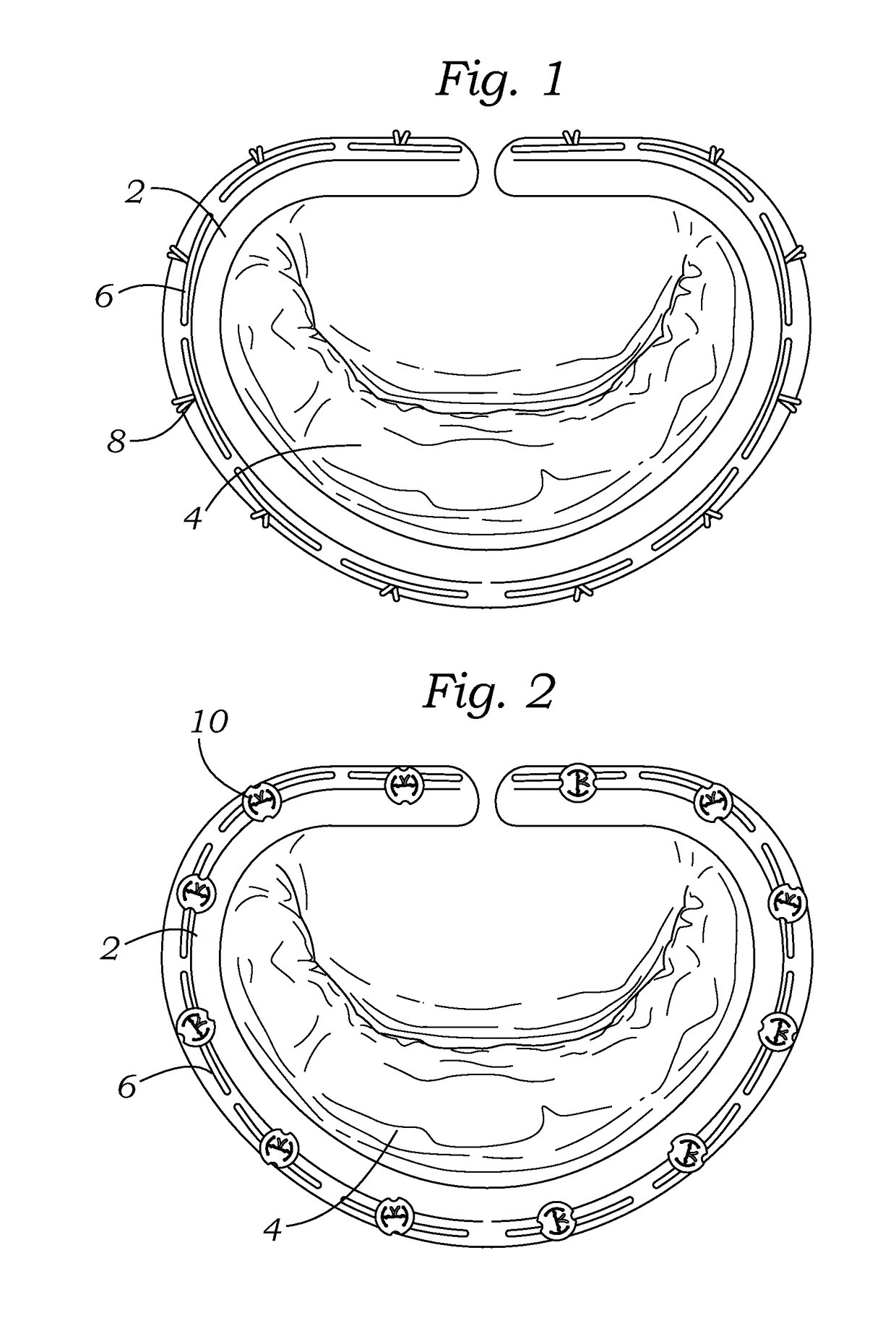
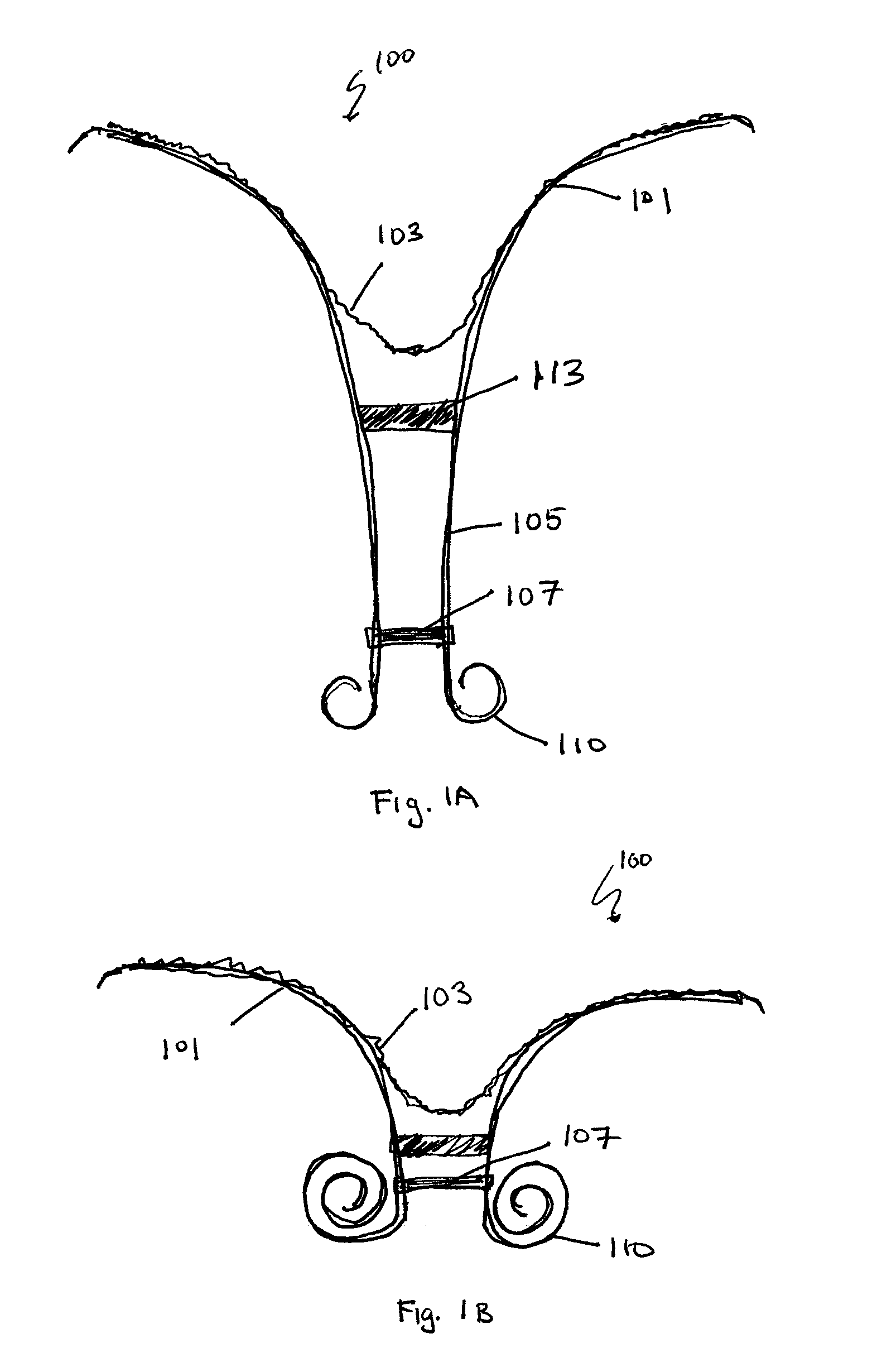
Retrievable devices for improving cardiac function
InactiveUS20090054723A1Shortening of regionShortening length of stemHeart valvesSurgical needlesBiomedical engineeringCardiac implanted
Owner:EDWARDS LIFESCIENCES CORP
Ventricular volume reduction
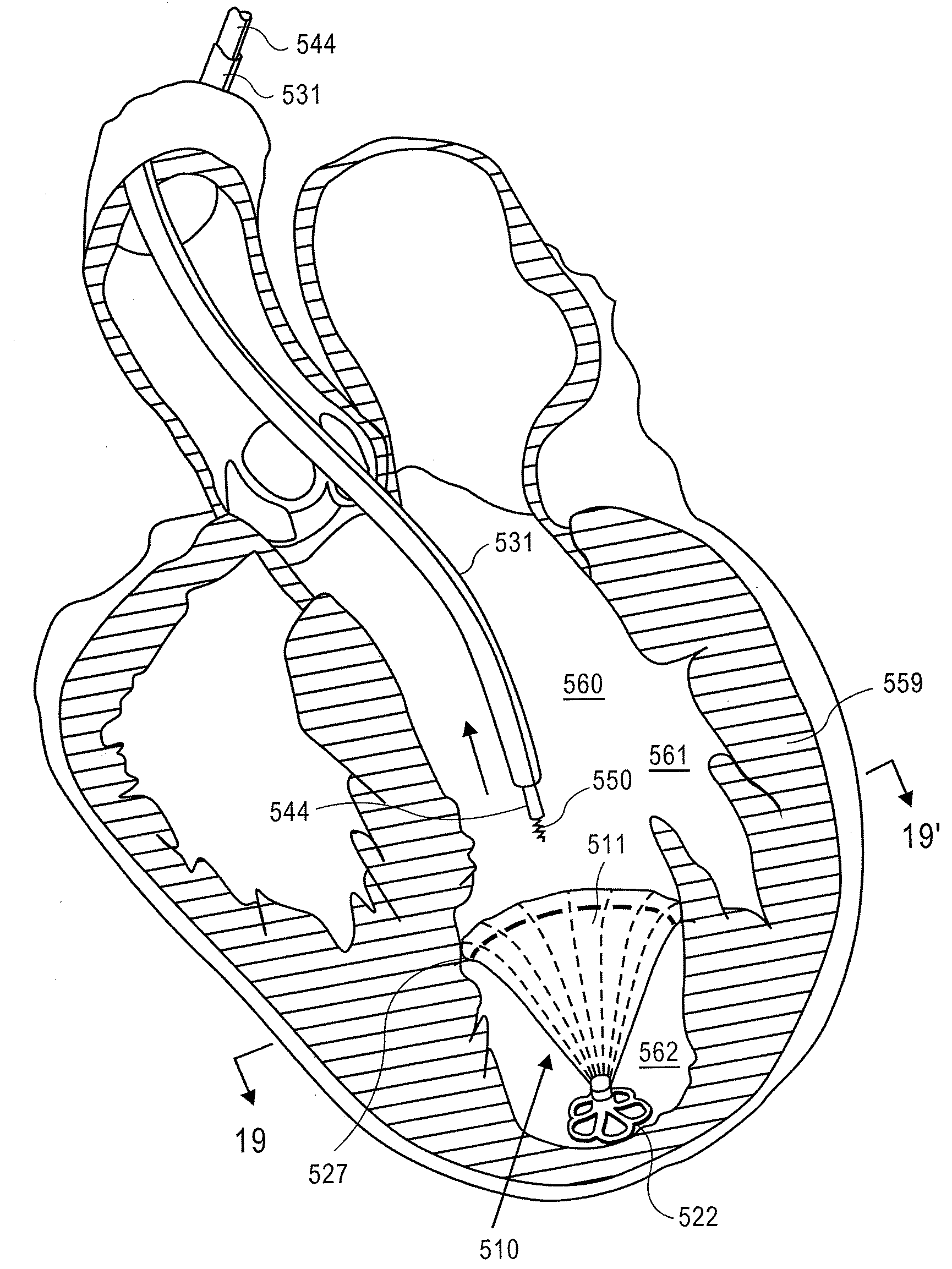
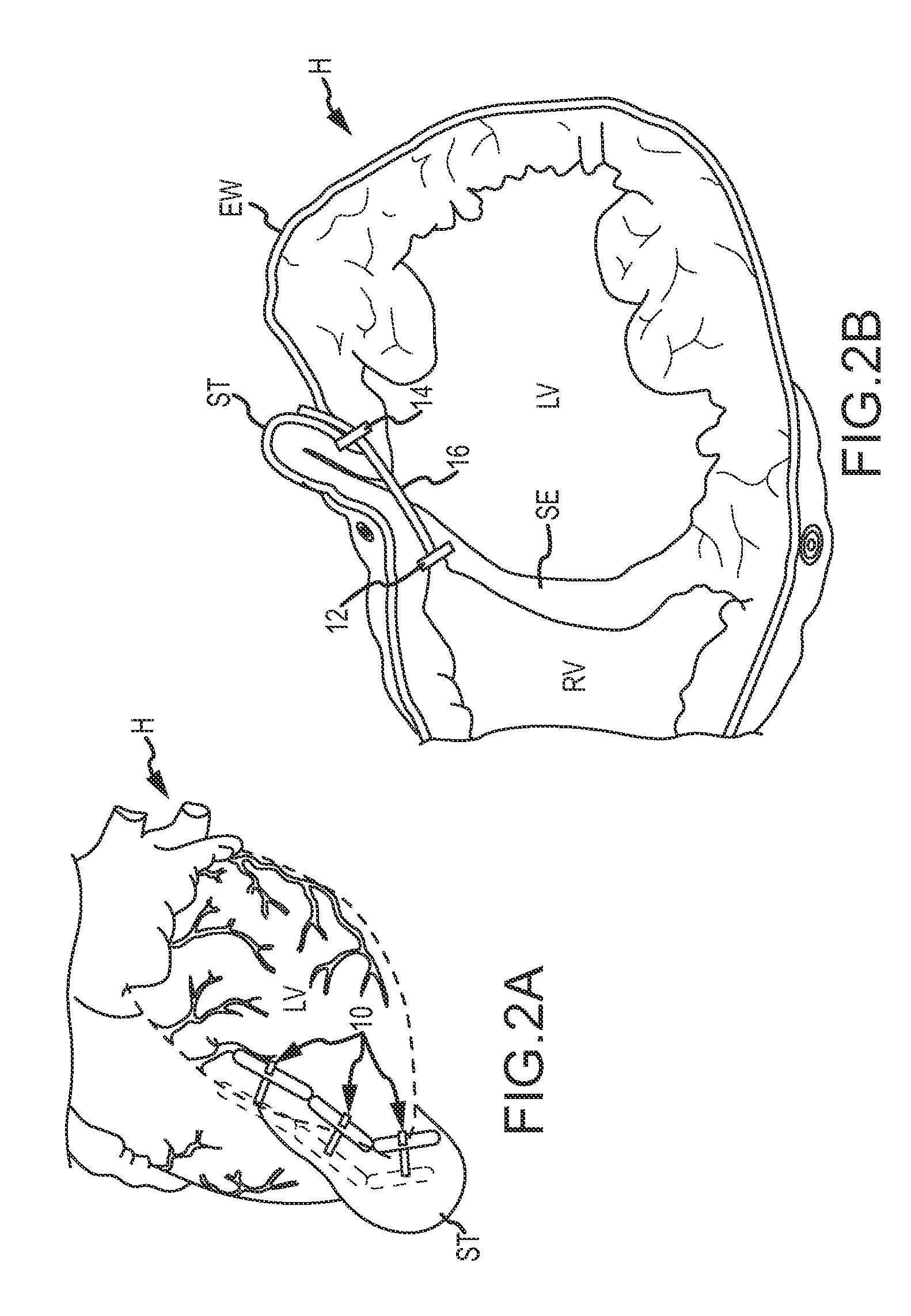
Devices and systems including implants (which may be removable) and methods of using them for reducing ventricular volume. The implants described herein are cardiac implants that may be inserted into a patient's heart, particularly the left ventricle. The implant may support the heart wall, or may be secured to the heart wall. The implants are typically ventricular partitioning device for partitioning the ventricle into productive and non-productive regions in order to reduce the ventricular volume.
Owner:EDWARDS LIFESCIENCES CORP
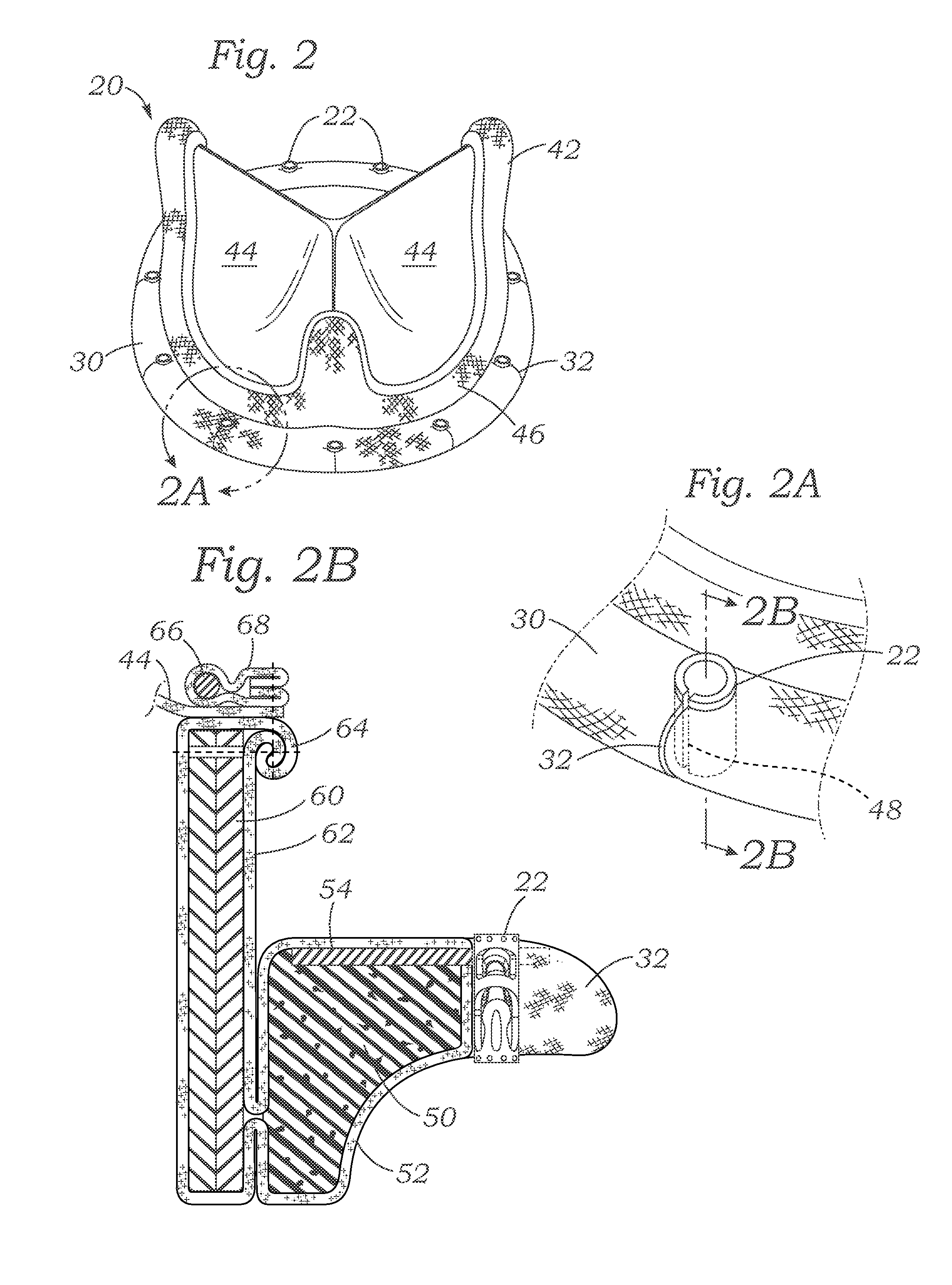
Cardiac implant with integrated suture fasteners
ActiveUS20140371842A1Improve ease of implantationEasy to deploySuture equipmentsAnnuloplasty ringsValved conduitProsthesis
A cardiac implant system including a cardiac implant such as an annuloplasty ring, a prosthetic heart valve, or a valved conduit pre-assembled at the time of manufacture with devices for securing the implant to a heart valve annulus using knotless suture fasteners. The knotless suture fasteners may be embedded within a pliant sealing edge of the cardiac implant, or they may be positioned adjacent to the sealing edge. The knotless suture fasteners are spring-biased so as to grip onto annulus anchoring sutures pass to therethrough upon removal of a restraining device, such as a hypotube inserted within the suture fasteners. Guide tubes are assembled in line with the suture fasteners to permit introduction of suture snares that pass through the suture fasteners and through the sealing edge to facilitate capture of the pre-installed annulus anchoring sutures.
Owner:EDWARDS LIFESCIENCES CORP
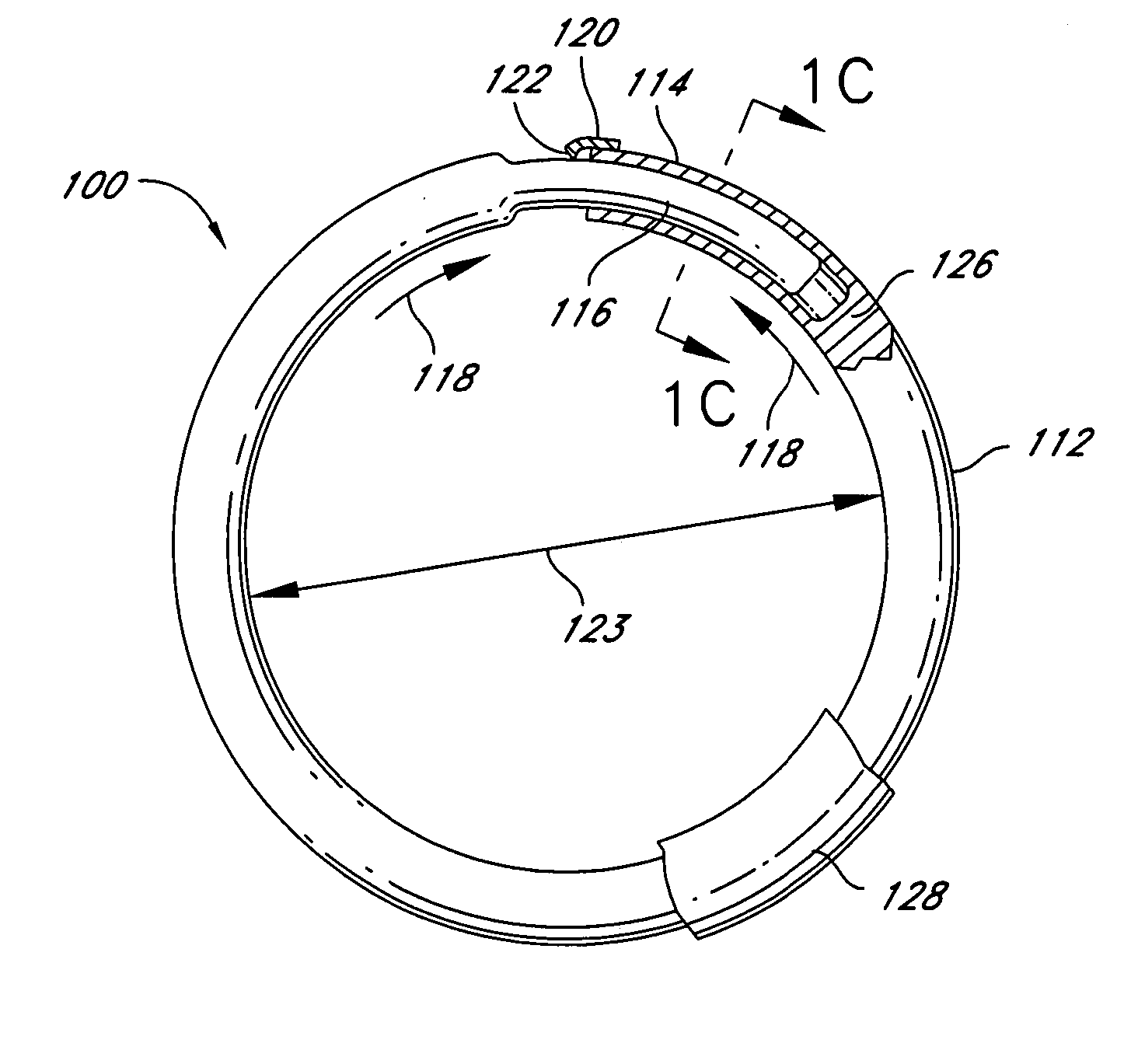
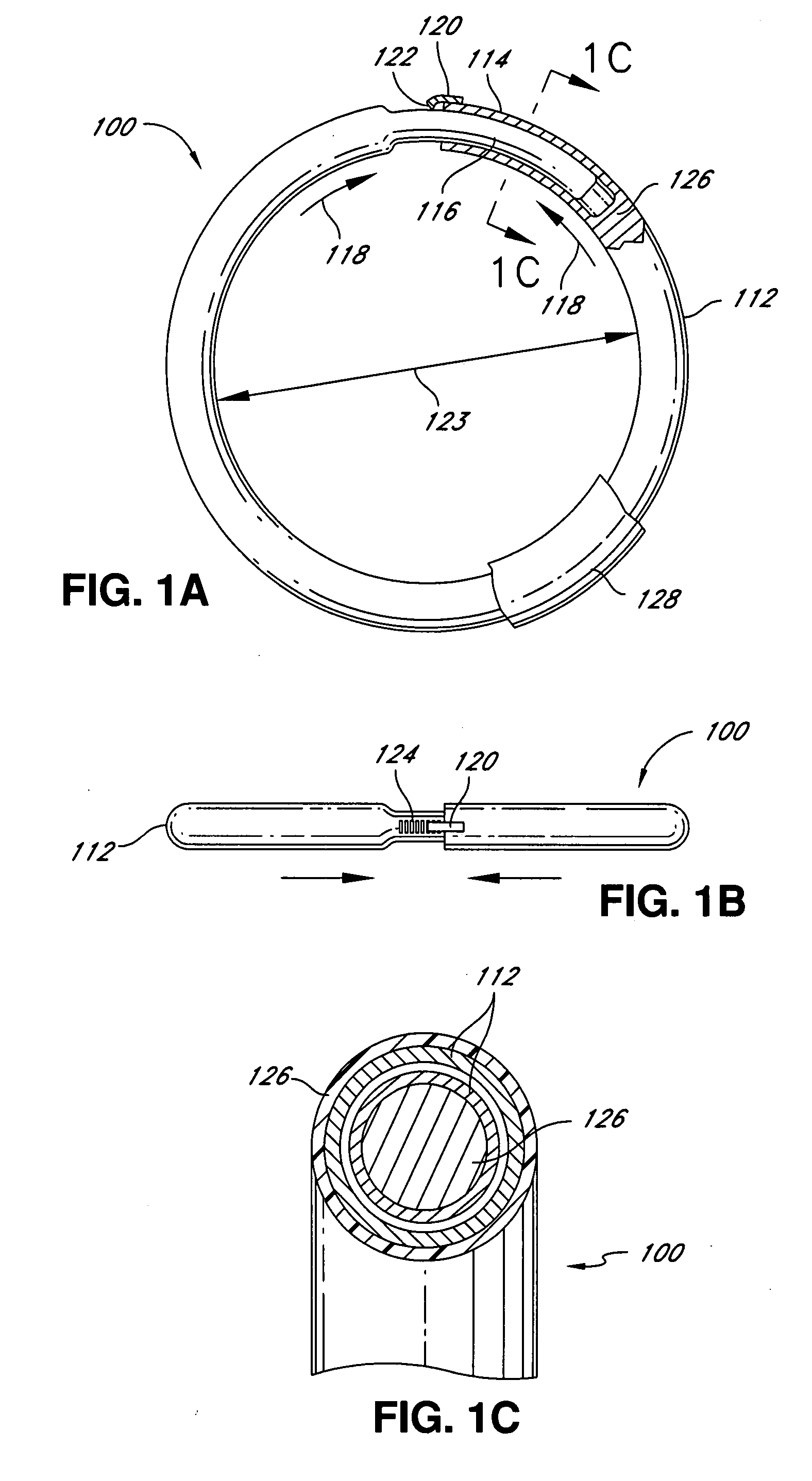
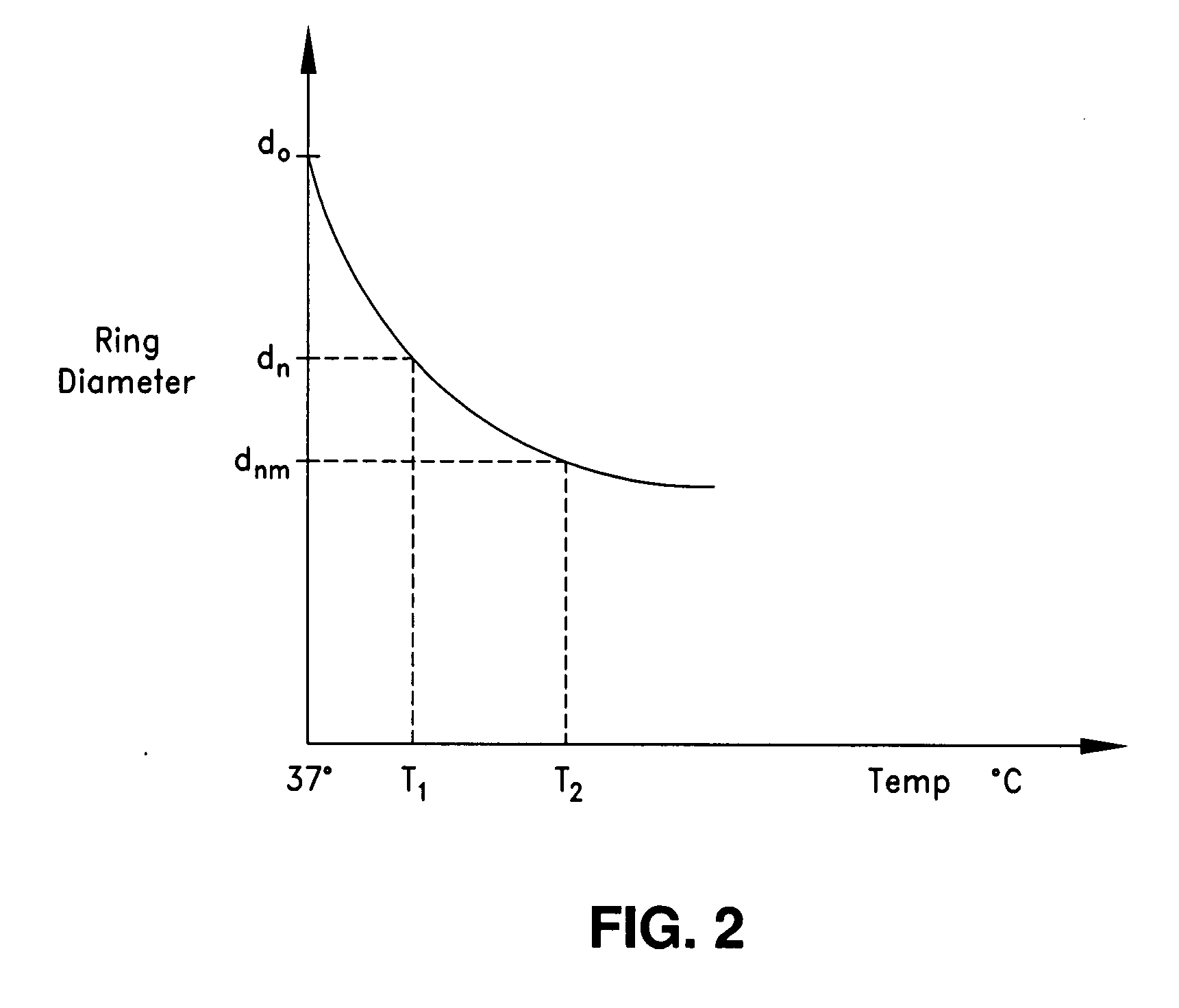
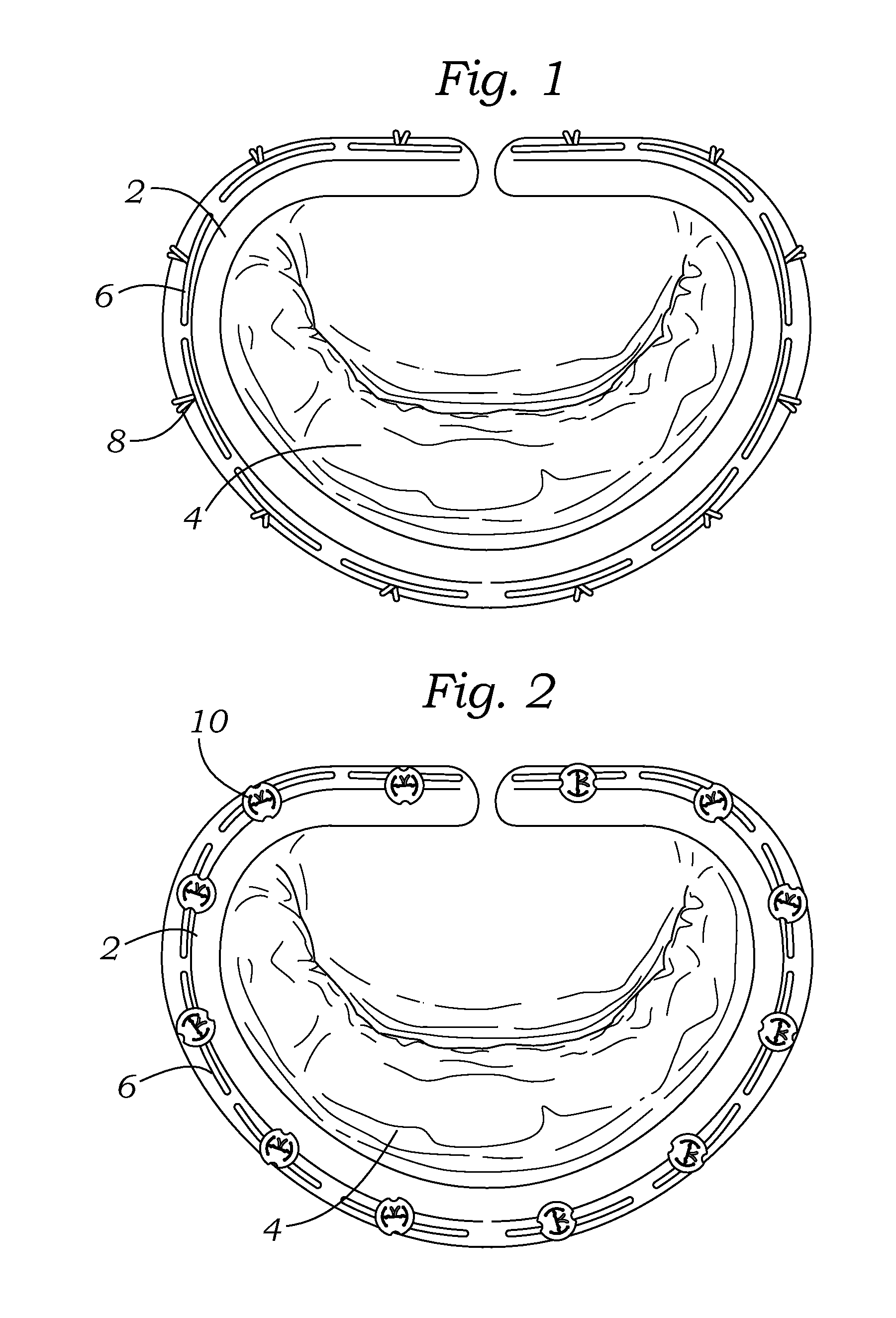
Intraoperative and post-operative adjustment of an annuloplasty ring
An intraoperative adjustment device is described. In some embodiments, the device includes an elongate body including a proximal end and a distal end, the distal end configured to penetrate an outer surface of an adjustable cardiac implant implanted in a patient's heart, and the proximal end and the distal end connected by at least one energy-transfer member. In some embodiments, the distal end includes at least one electrode coupled to the energy-transfer member and configured to deliver an activation energy to the adjustable cardiac implant. In some embodiments, the proximal end is configured to attach to an energy source that provides the activation energy. In some embodiments, the proximal end is configured to be located outside the patient's body while the distal end is coupled to the adjustable cardiac implant that is implanted in the patient's heart.
Owner:MICARDIA CORP
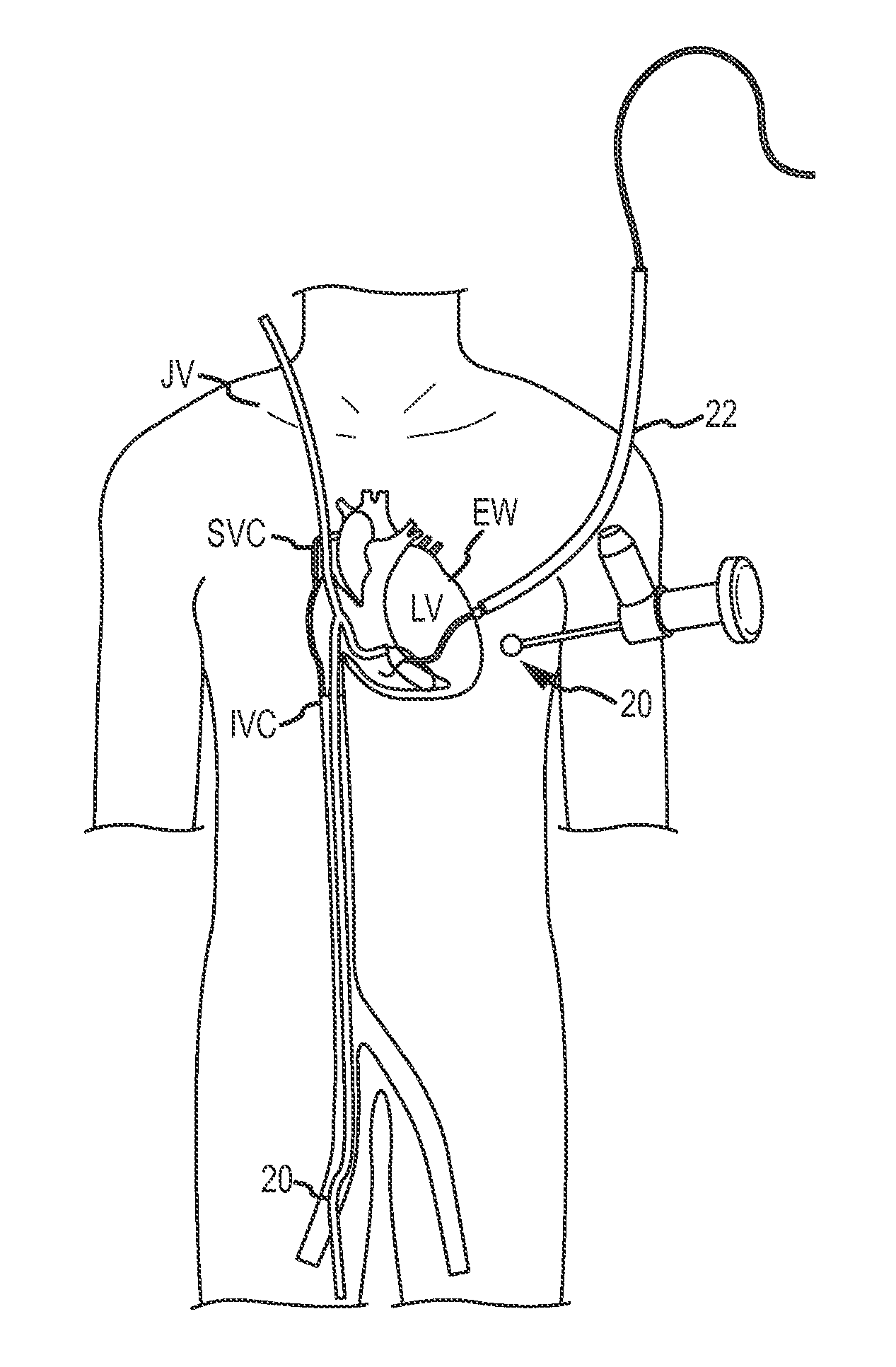
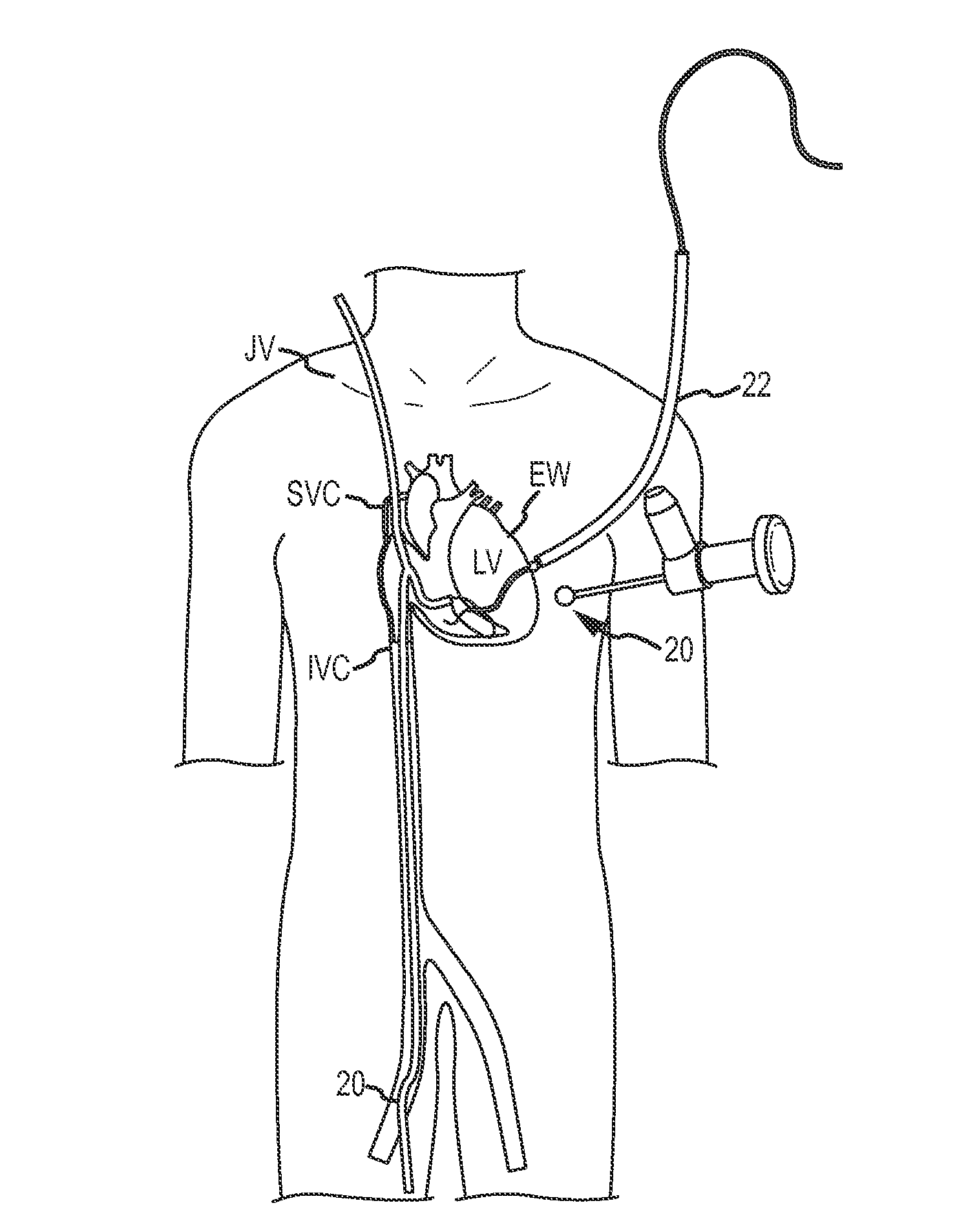
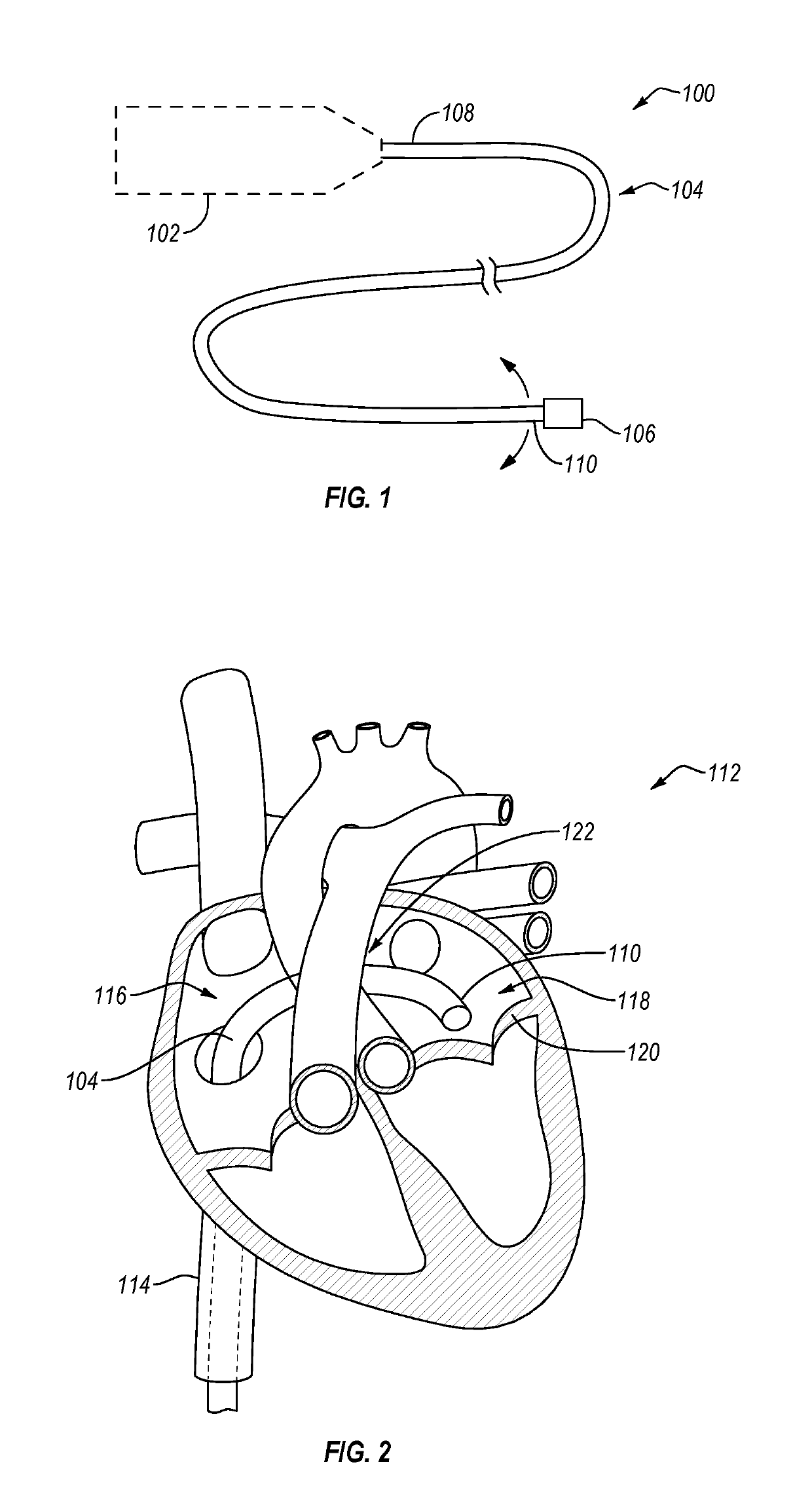
Over-the-wire cardiac implant delivery system for treatment of chf and other conditions
Medical devices, systems, and methods reduce the distance between two locations in tissue in a minimally invasive manner, often for treatment of congestive heart failure. In one embodiment, an anchor of an implant system may, when the implant system is fully deployed, reside within the right ventricle in engagement with the ventricular septum. A tension member may extend from that anchor through the septum and an exterior wall of the left ventricle to a second anchor disposed along an epicardial surface of the heart. Deployment of the anchor within the right ventricle may be performed by inserting a guidewire through the septal wall into the right ventricle. The anchor may be inserted into the right ventricle over the guidewire and through a lumen of a delivery catheter. Delivering the anchor over the guidewire may provide improved control in the delivery and placement of the anchor within the right ventricle.
Owner:BIOVENTRIX A CHF TECH
Cardiac implant migration inhibiting systems
ActiveUS20130090672A1Reduce distanceEasy to controlSuture equipmentsHeart valvesSeptal wallCongestive heart failure chf
Medical devices, systems, and methods reduce the distance between two locations in tissue, often for treatment of congestive heart failure. In one embodiment an anchor of an implant system may reside within the right ventricle in engagement with the ventricular septum. A tension member may extend from that anchor through the septum and an exterior wall of the left ventricle to a second anchor disposed along an epicardial surface. Deployment of the anchor within the right ventricle may be performed by inserting a guidewire through the septal wall into the right ventricle. The anchor may be inserted into the right ventricle over the guidewire and through a lumen of a catheter. An anchor force may be applied within a desired range to secure the anchors about the septum and epicardial surface. The anchor force may inhibit migration of the anchors relative to the septum and epicardial surface.
Owner:BIOVENTRIX A CHF TECH
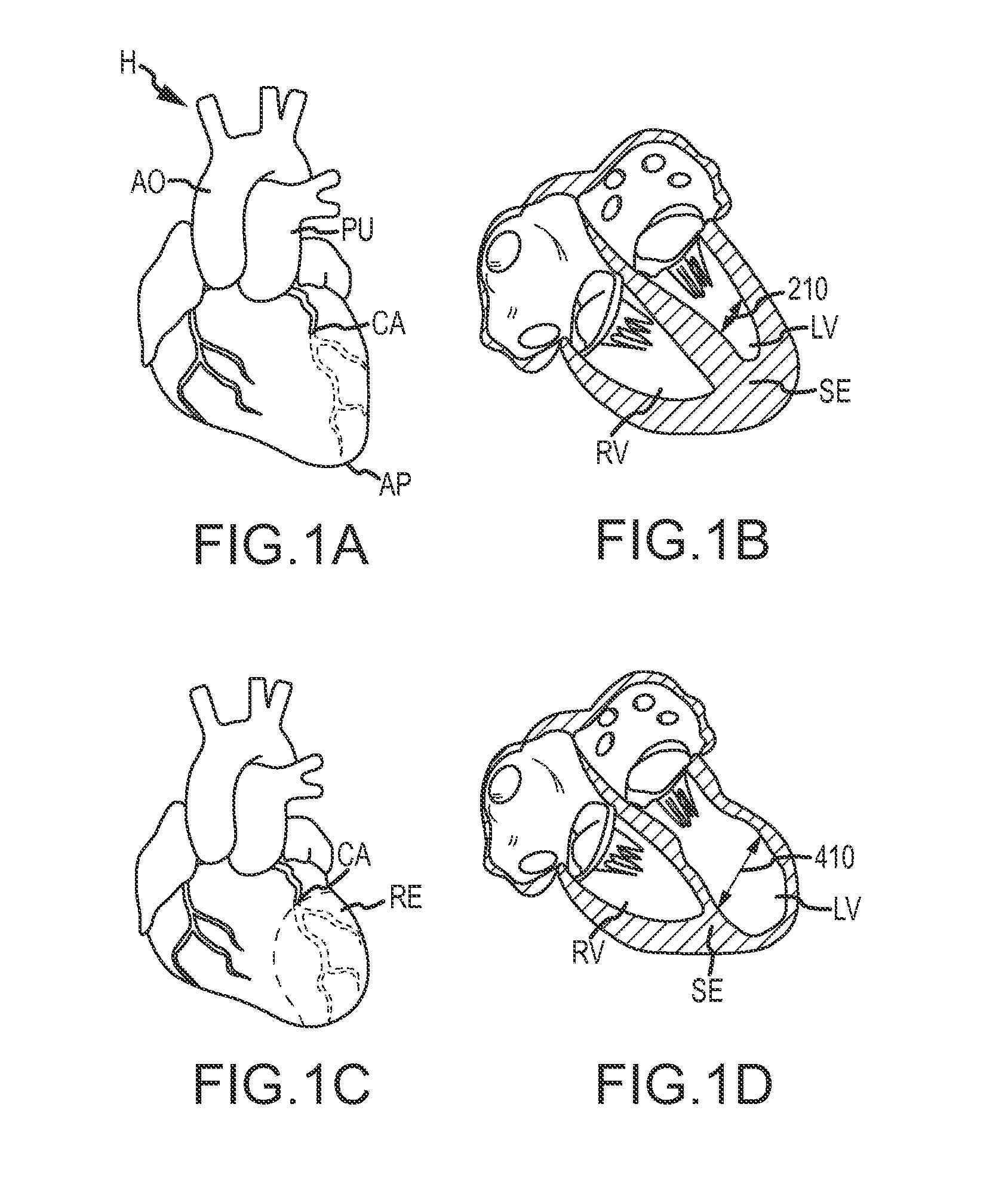
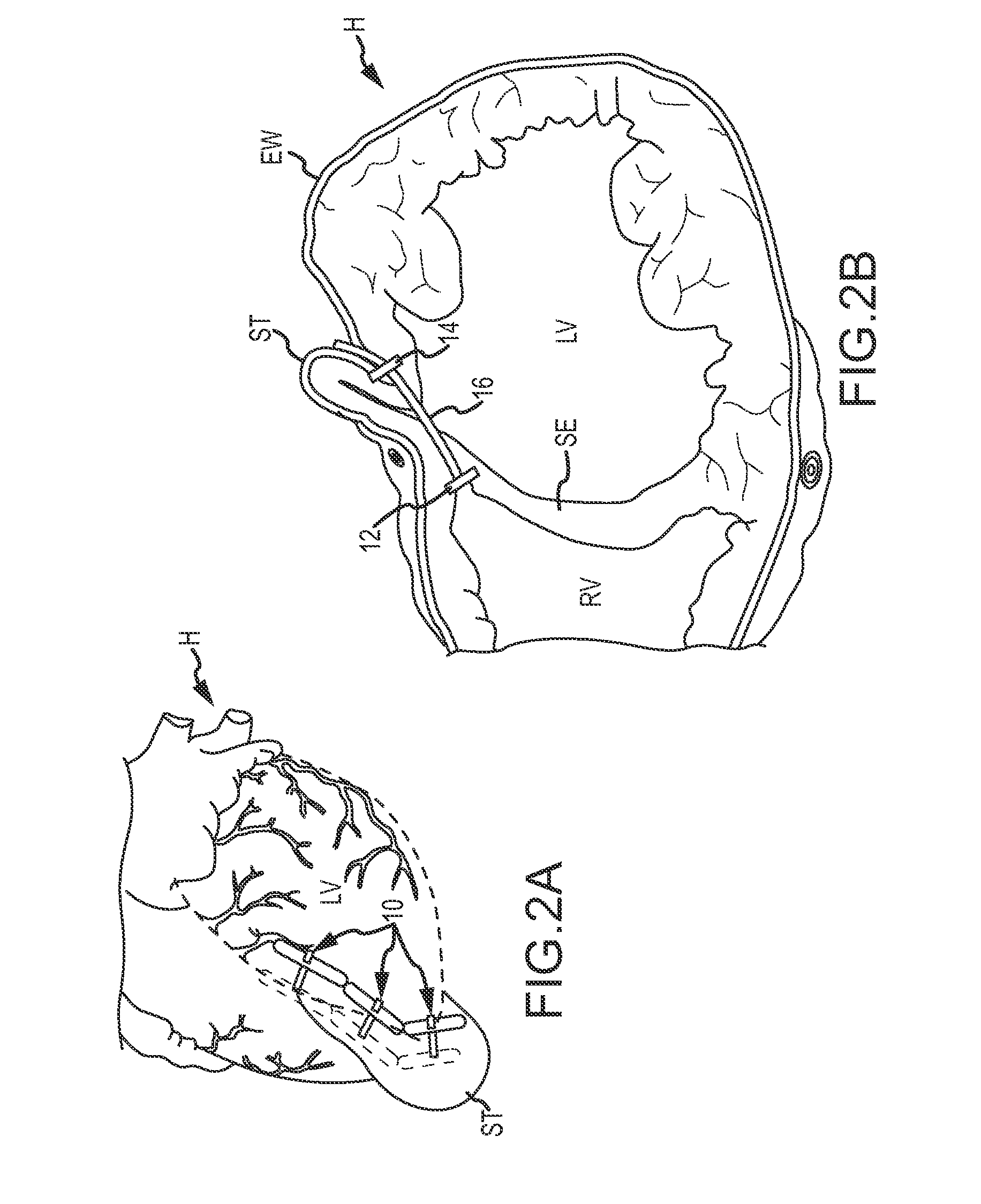
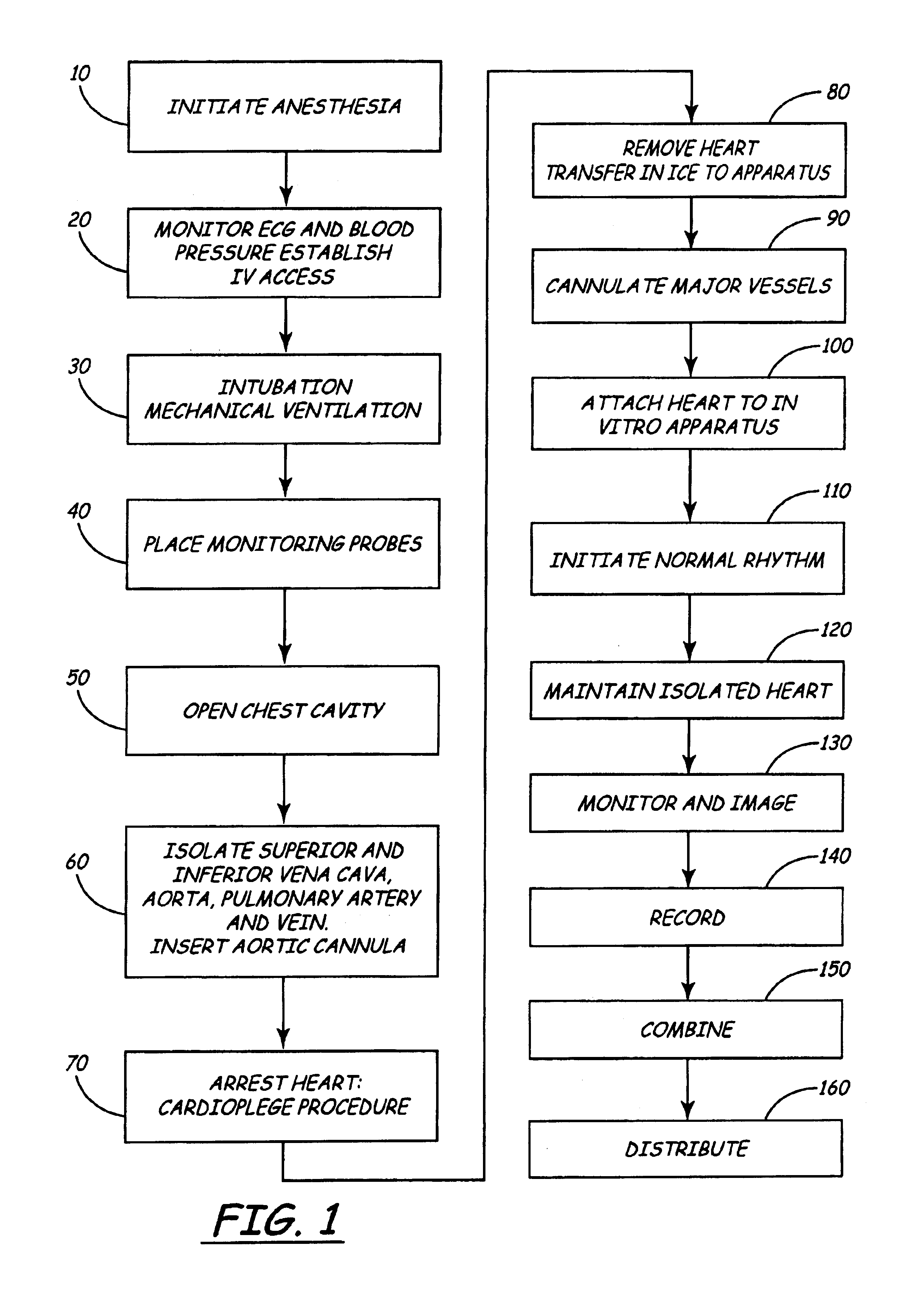
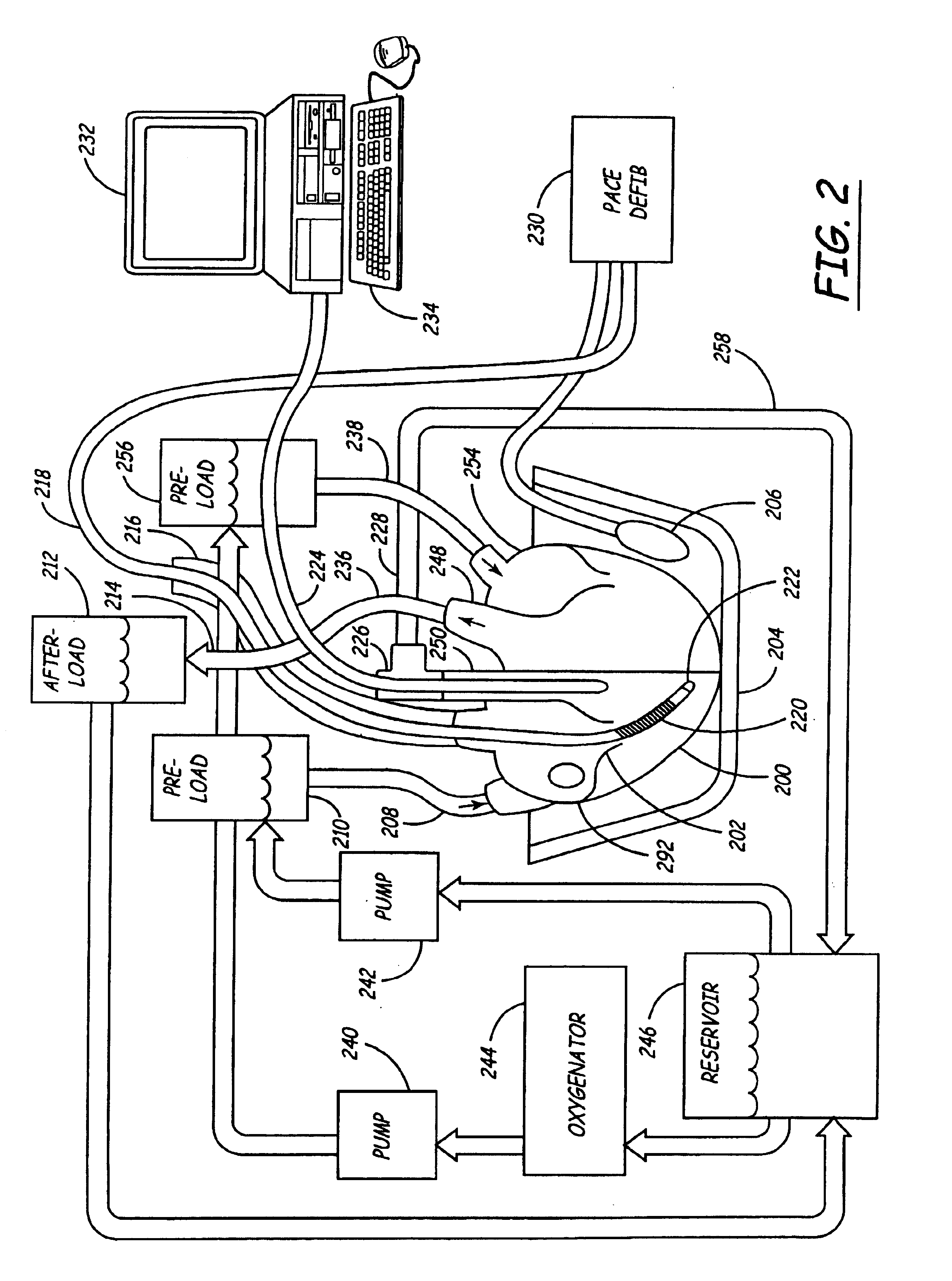
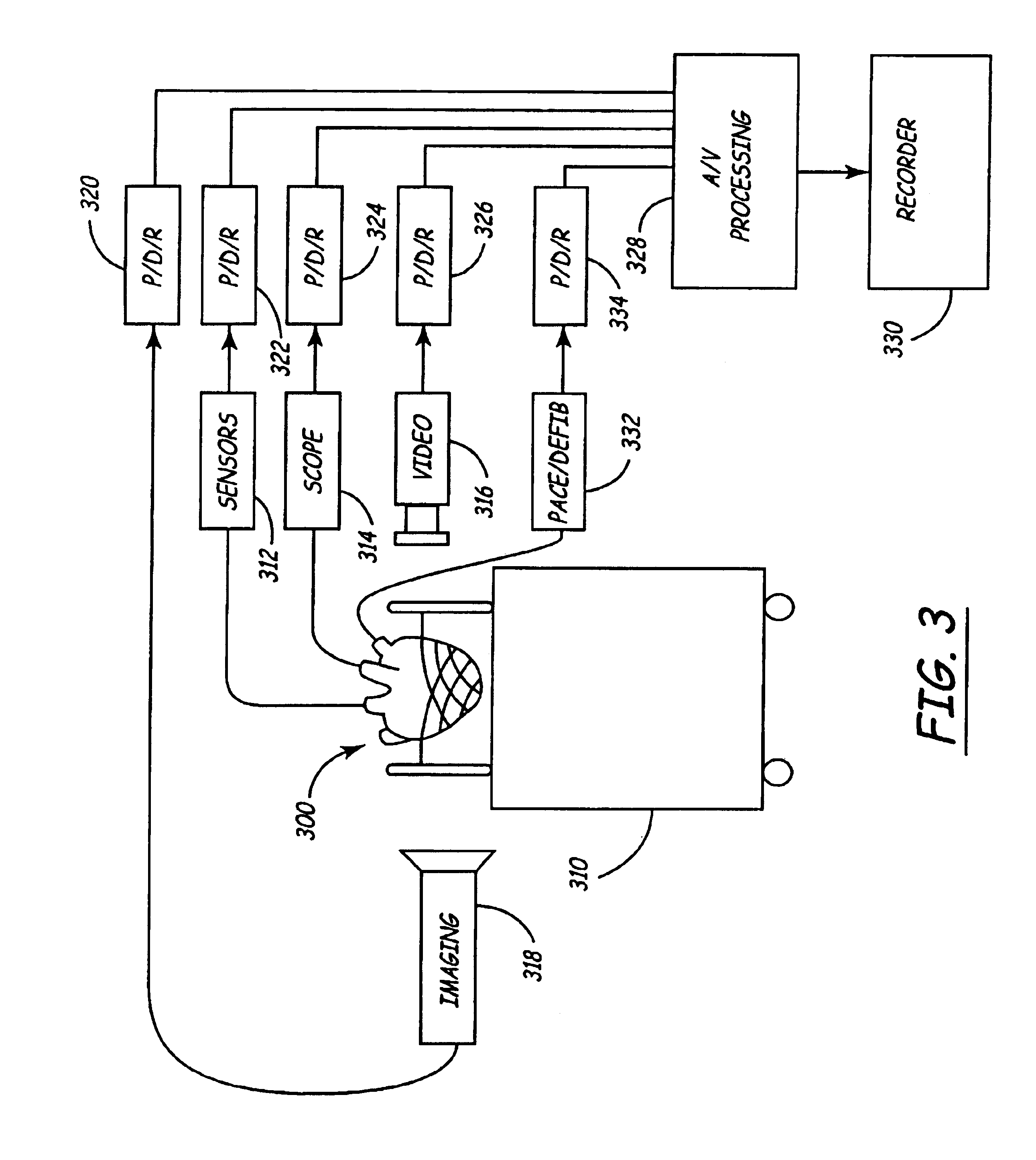
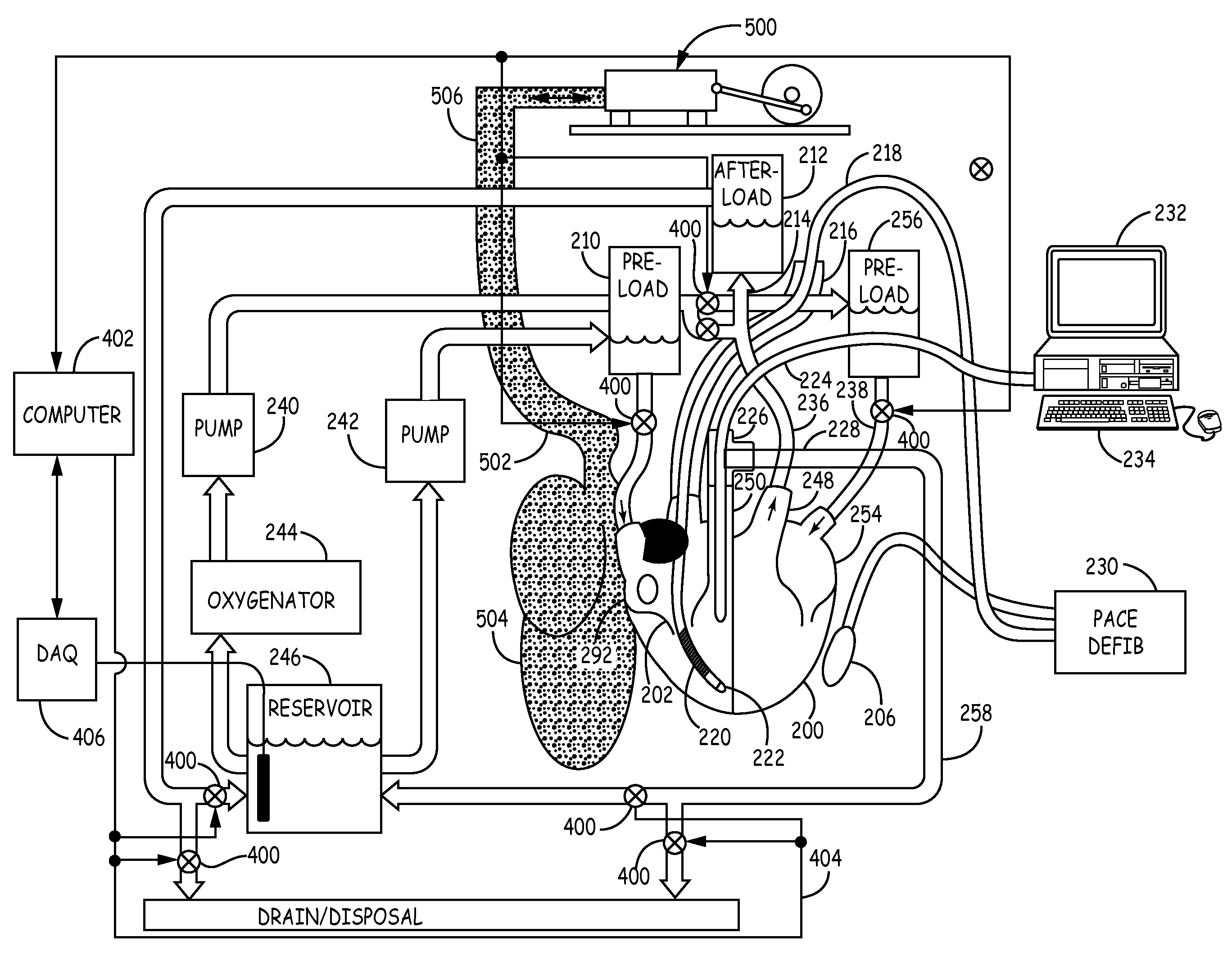
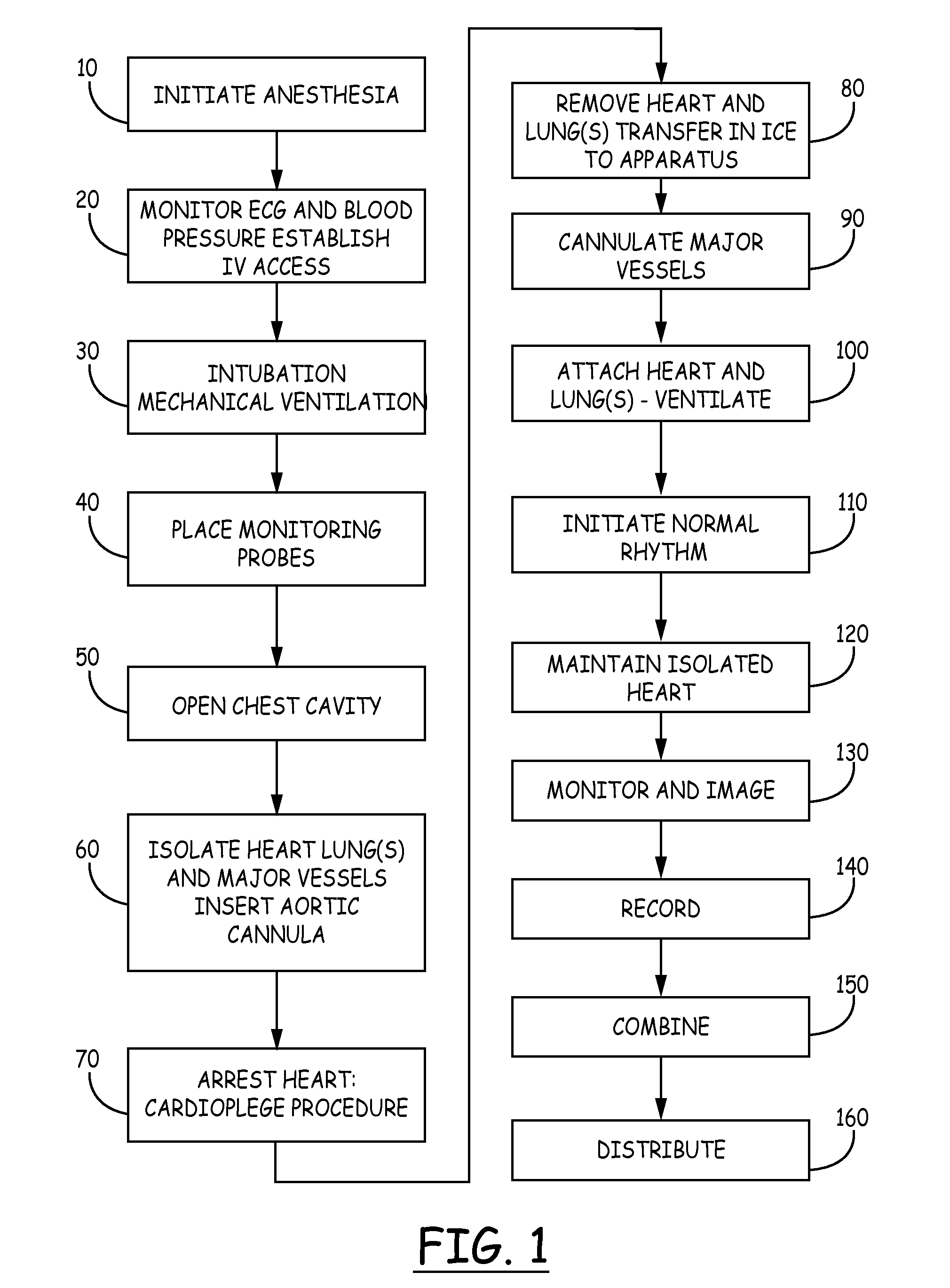
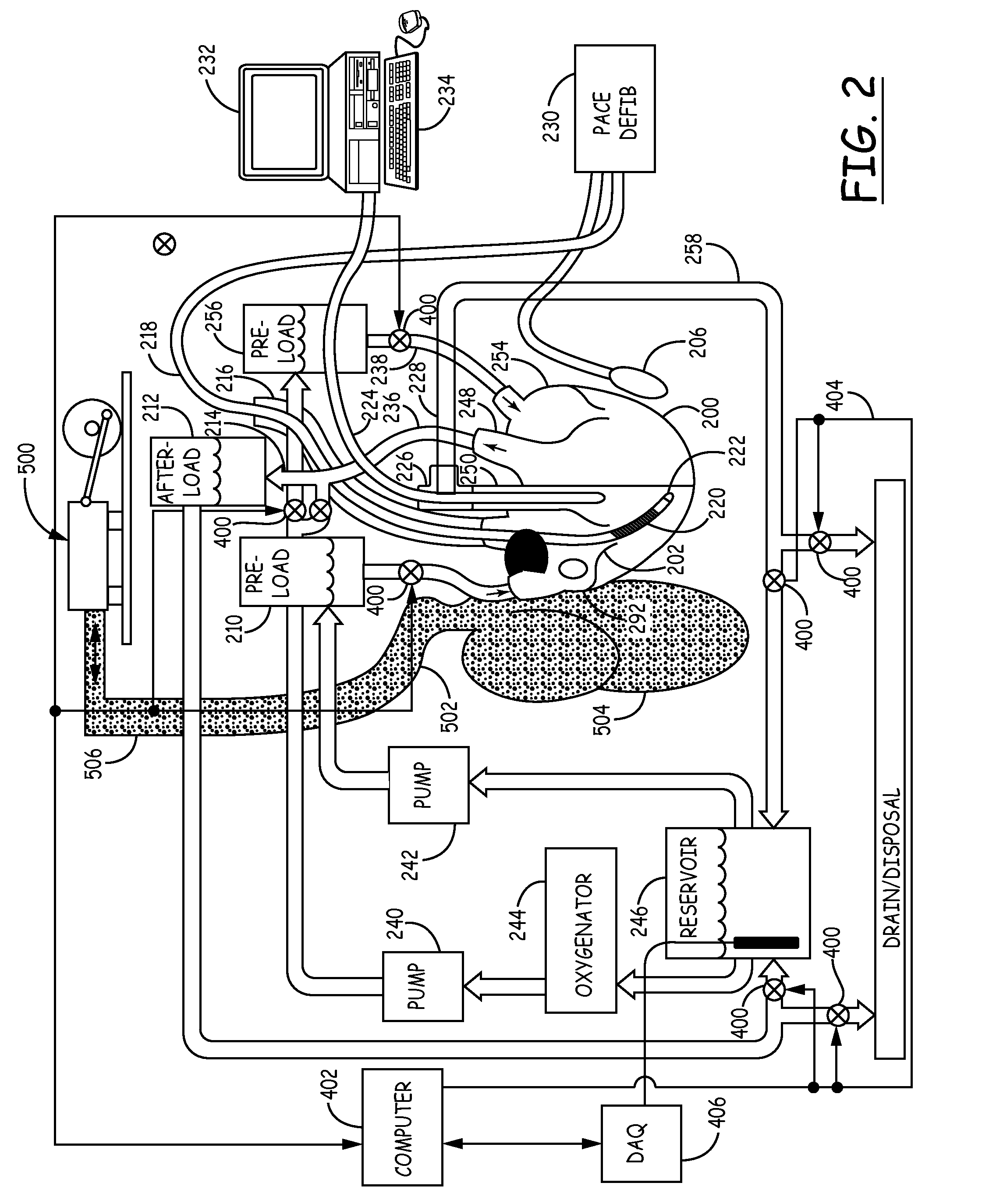
Isolated perfused heart preparation and method of use
InactiveUS7045279B1Easy to produceMaintain activityDead animal preservationCatheterKrebs bufferMedical treatment
An isolated heart preparation in which essentially normal pumping activity of all four chambers of the heart is preserved, allowing for the use of the preparation in conjunction with investigations of electrode leads, catheters, cardiac implants and other medical devices intended to be used in or on a beating heart. The preparation may also be employed to investigate heart functions, in the presence or absence of such medical devices. In order to allow for visualization of heart structures and devices located within the chambers of the heart, a clear perfusate such as a modified Krebs buffer solution with oxygenation is circulated through all four chambers of the heart and the coronary vasculature. The preparation and recordings of the preparation may be used in conjunction with the design, development and evaluation of devices for use in or on the heart, as well as for use as an investigational and teaching aid to assist physicians and students in understanding the operation of the heart.
Owner:MEDTRONIC INC
Method and device for improving cardiac function
InactiveUS7798953B1Facilitate invasive procedureImprove heart functionDiagnosticsSurgeryVeinEffective volume
In a method for improving cardiac performance, a compressive cardiac implant is passed through an anterior intraventricular vein of a patient's heart to a position proximate an apex or lower end of a left ventricle of the patient's heart. A distal end portion of the compressive cardiac implant is moved from the anterior intraventricular vein into the patient's left ventricle and thereafter passed through a septum of the patient's heart and into a right ventricle thereof. The distal end portion of the compressive cardiac implant is then engaged with the septum, and the compressive cardiac implant is operated to compress the apex or lower end of the patient's left ventricle so as to reduce the effective volume of the left ventricle.
Owner:WILK PATENT
Heart-lung preparation and method of use
InactiveUS20140370490A1Easy to produceMaintain activityDead animal preservationEducational modelsPulmonary vasculatureLung structure
An isolated heart or heart-lung preparation in which essentially normal pumping activity of all four chambers of the heart is preserved, allowing for the use of the preparation in conjunction with investigations of electrode leads, catheters, ablation methods, cardiac implants and other medical devices intended to be used in or on a beating heart. The system can be designed to be used within a Magnetic Resonance Imaging (MRI) unit or a X-ray computed tomography (CT) scanner. The preparation may also be employed to investigate heart and lung functions, in the presence or absence of such medical devices. In order to allow comparative imaging visualizations of either or simultaneously the heart and / or lung structures and devices located within the chambers of the heart or vessels or bronchi within the lungs, a clear perfusate such as a modified Krebs buffer solution with oxygenation is circulated through all four chambers of the heart and thus the coronary and / or pulmonary vasculatures. A ventilator with intubation tube can be used to inflate / deflate the lungs and / or provide oxygen to the isolated organs. The preparation and recordings of the preparation may be used in conjunction with the design, development and evaluation of devices for use in or on the heart and / or lungs, as well as for use as an investigational and teaching aid to assist physicians and students in understanding the operation of the cardiopulmonary system.
Owner:MEDTRONIC INC
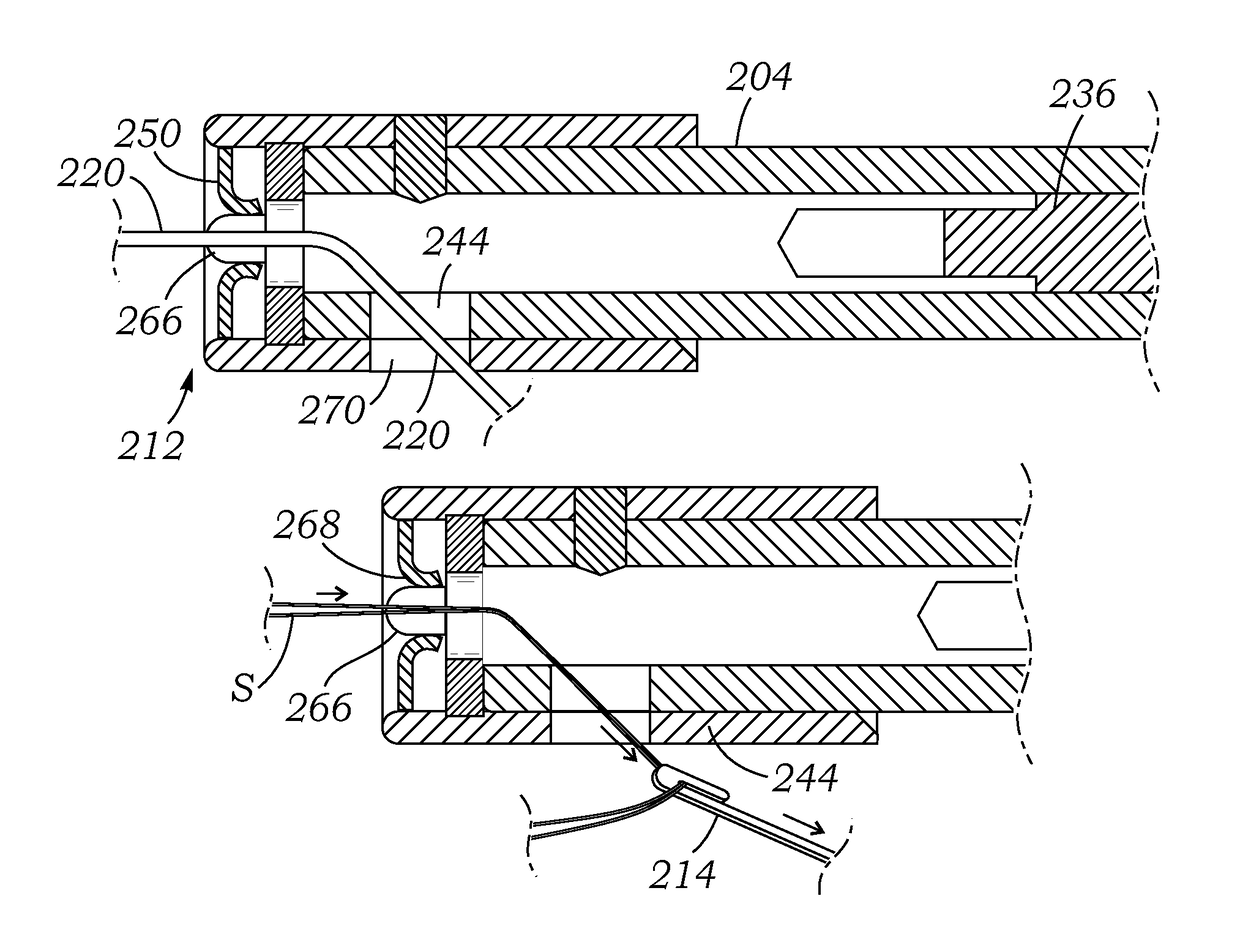
Knotless suture fastener installation system
A knotless suture fastener installation system for securing medical devices such as cardiac implants. The knotless suture fasteners may be spring-biased so as to grip onto sutures passed therethrough. The system includes a fastener deployment tool with a proximal handle and a distal shaft to which a fastener cartridge attaches. A plurality of disposable cartridges are sequentially attached to the end of the deployment tool and used to secure the medical implant one fastener at a time. The deployment tool may also cut the sutures being fastened.
Owner:EDWARDS LIFESCIENCES CORP
Cardiac implant configured to receive a percutaneous prosthetic heart valve implantation
The invention is a cardiac implant, and associated methods therefore, configured to repair and / or replace a native heart valve, and having a support frame configured to be reshaped into an expanded / changed form in order to receive and / or support an expandable prosthetic heart valve therein. The implant may be configured to have a generally rigid and / or expansion-resistant configuration when initially implanted to replace / repair a native valve (or other prosthetic heart valve), but to assume a generally non-rigid and / or expanded / expandable form when subjected to an outward force such as that provided by a dilation balloon. The implant may be configured to have a generally D-shaped configuration when initially implanted, but to assume a generally circular form when subjected to an outward force such as that provided by a dilation balloon.
Owner:EDWARDS LIFESCIENCES CORP
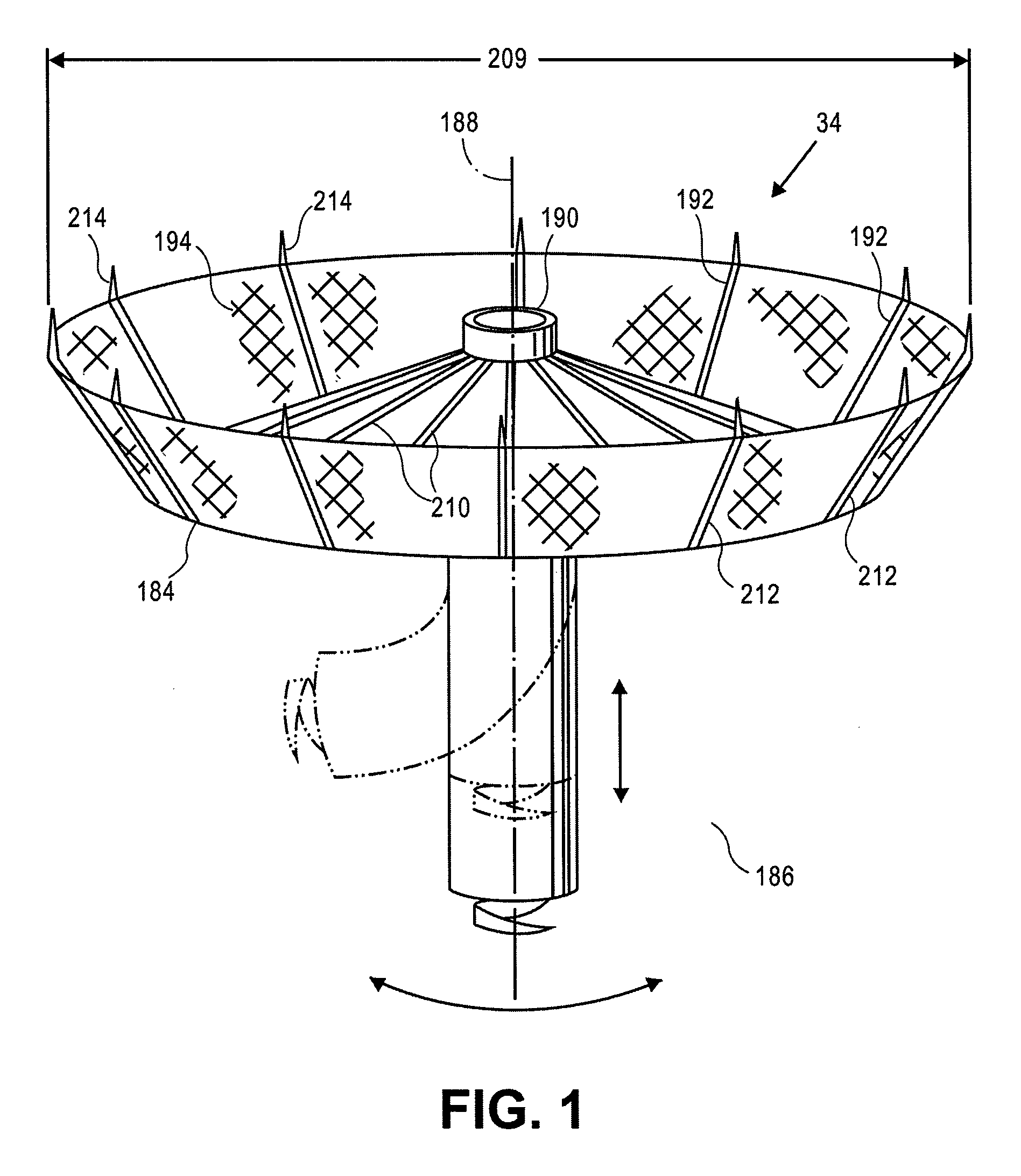
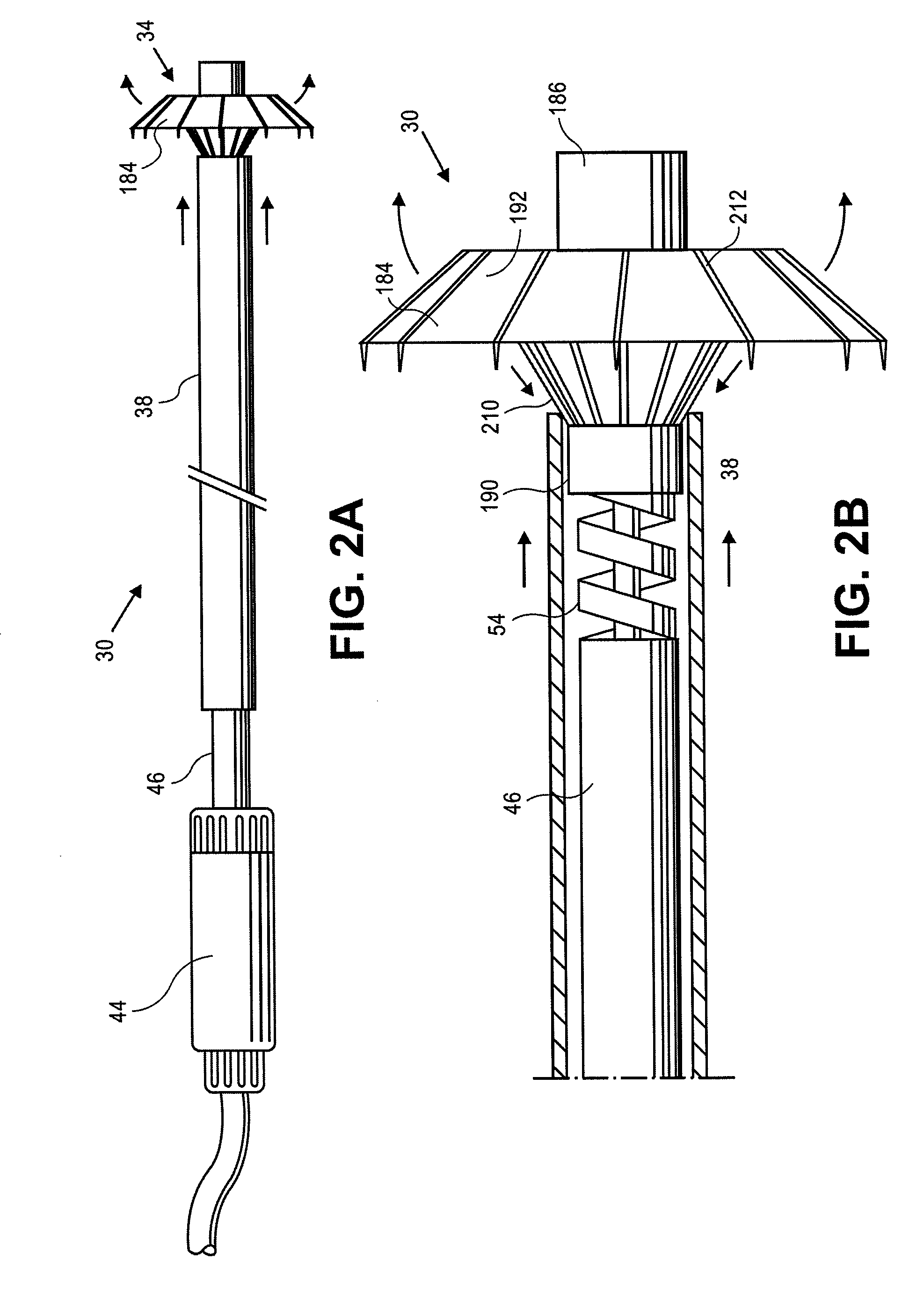
Retrievable cardiac devices
ActiveUS8246671B2Shorten the lengthShortening of regionSuture equipmentsHeart valvesCardiac deviceEndocrinology
Owner:EDWARDS LIFESCIENCES CORP
Devices and methods for delivering an endocardial device
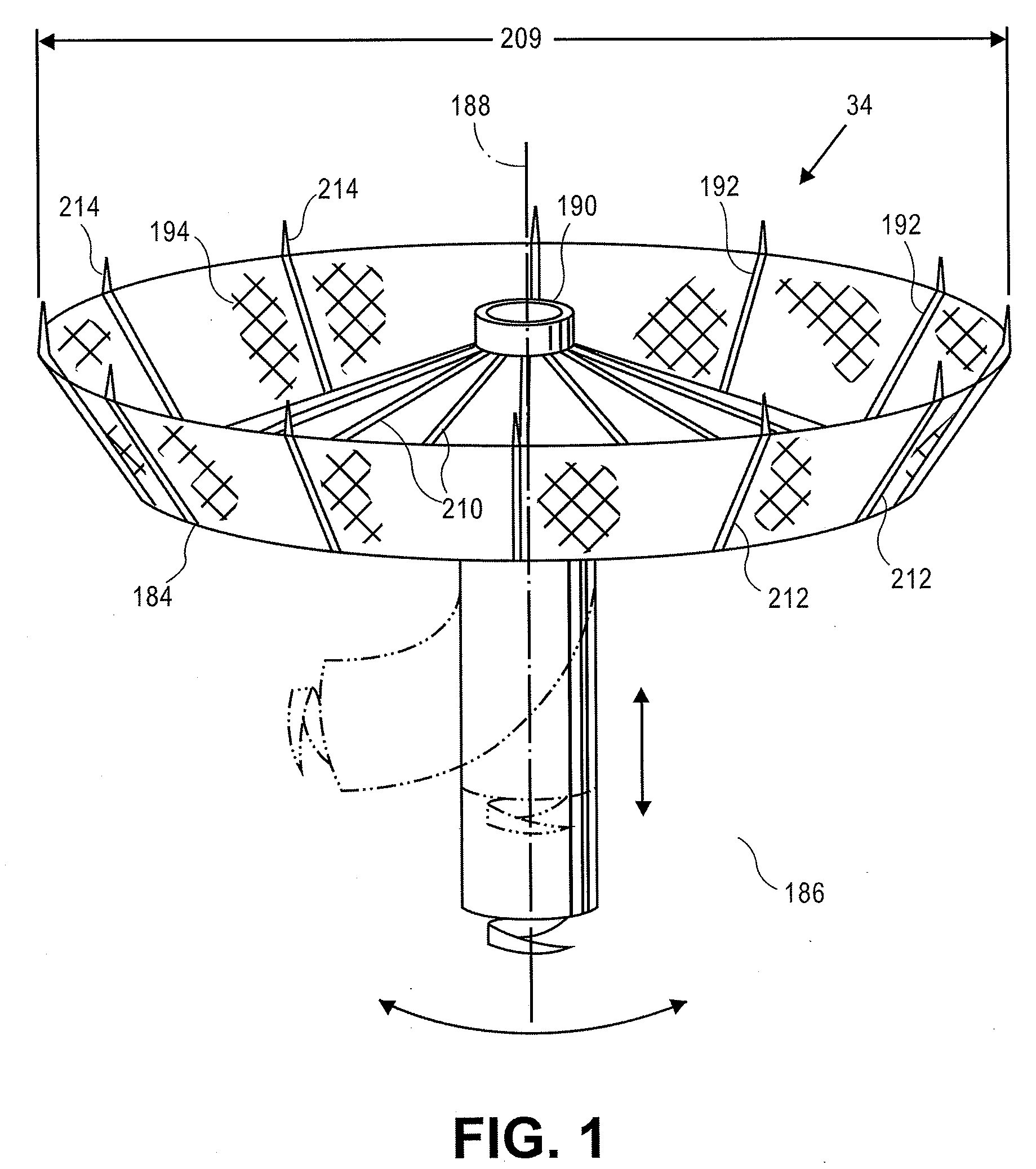
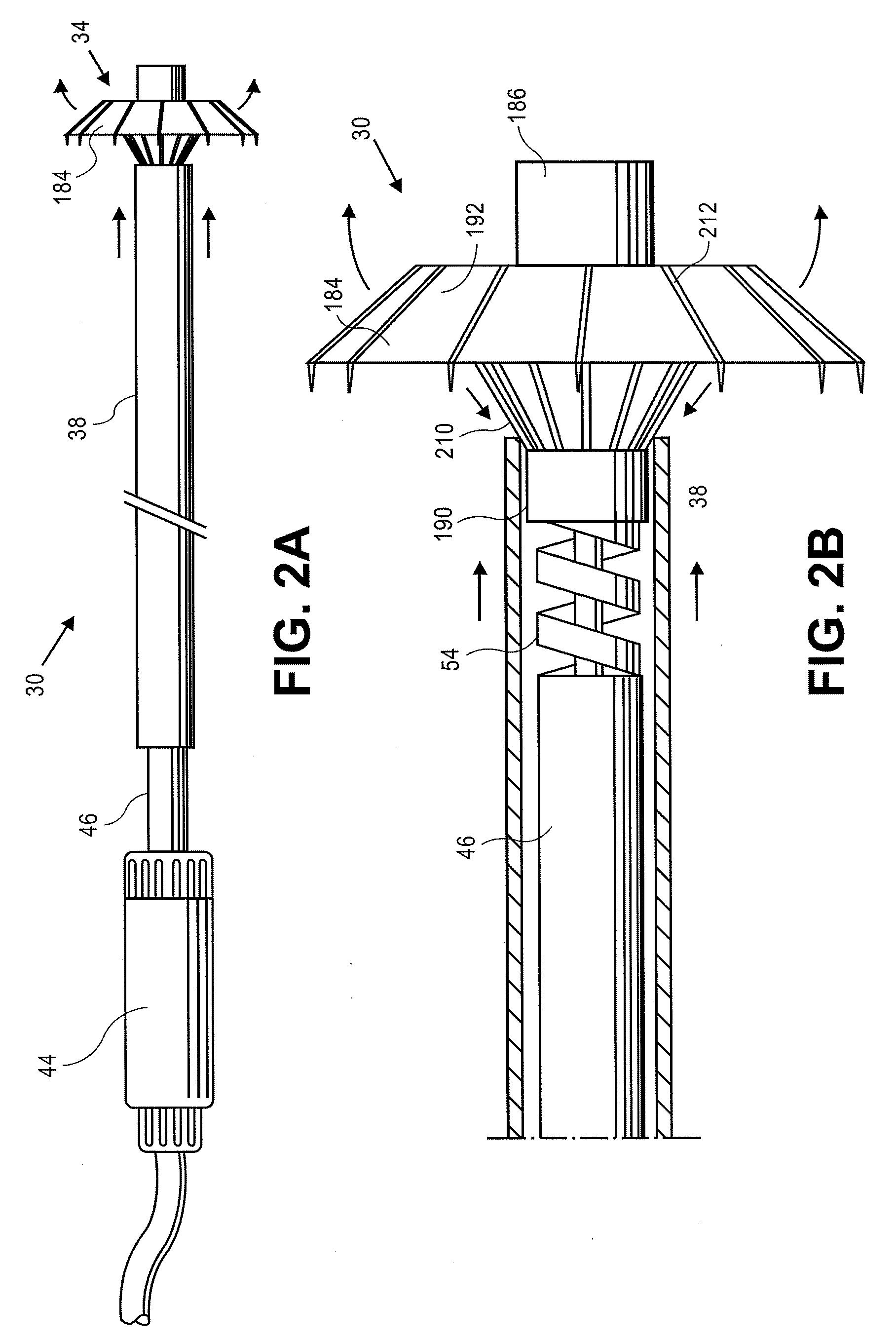
Apical reconstruction and support devices for use in a patient's ventricle include removable implants that may partition the ventricle. Such devices may be implanted using an applicator or system configured for inserting, repositioning and / or removing them. Described herein are applicators, systems, and methods of positioning, deploying and removing cardiac implants. The implants described herein may be inserted into a chamber of a patient's heart, particularly near the apex of the left ventricle, and may support the heart wall. Li some variations the implant is a ventricular partitioning device for partitioning the ventricle into productive and non-productive regions. The applicators may include an expandable member or members at the distal end of a guide to adjustably move the tip of the guide catheter within the ventricle before or during deployment of the implant from the distal end of the guide catheter. These applicators may displace trabeculations within the ventricle.
Owner:EDWARDS LIFESCIENCES CORP
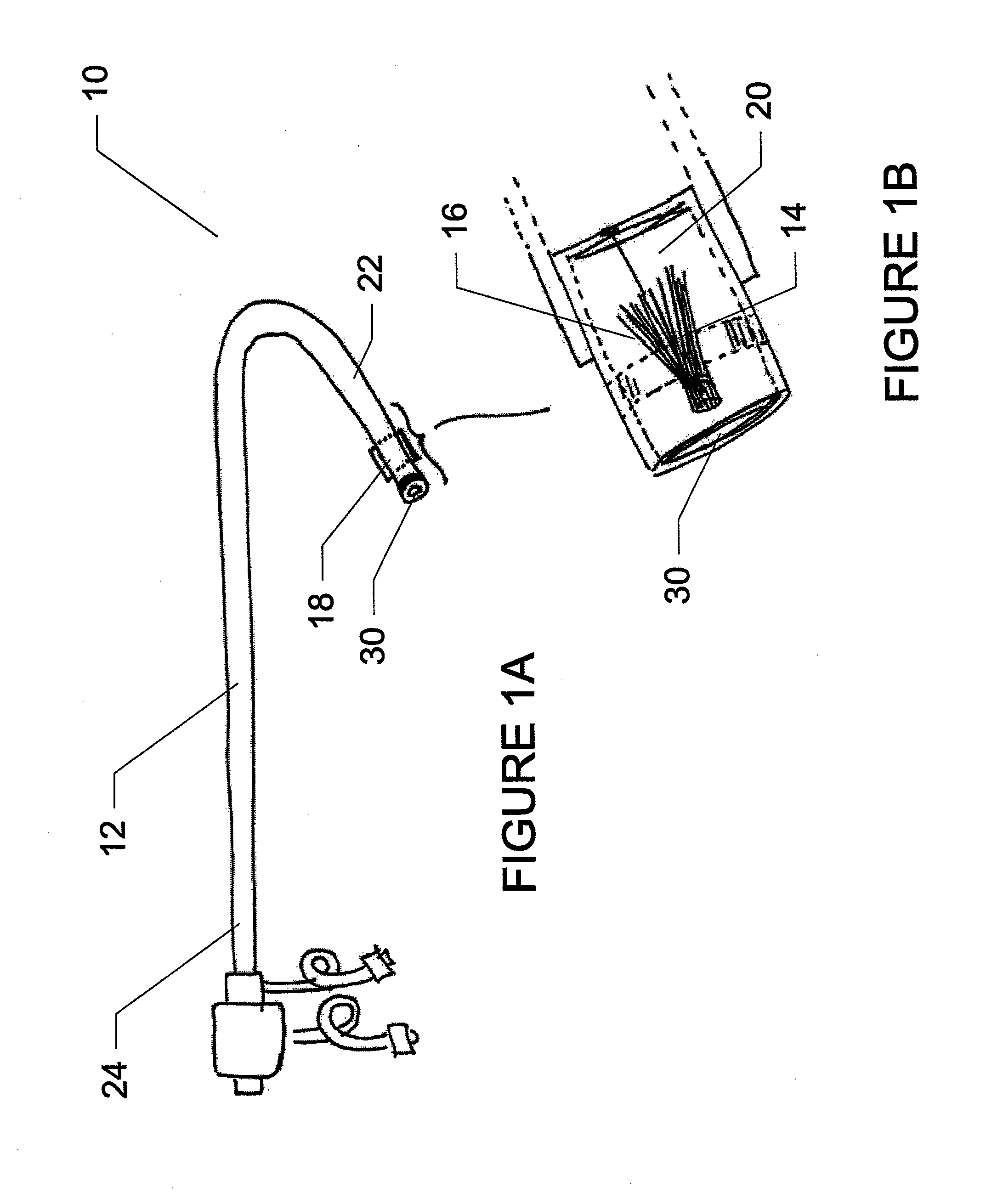
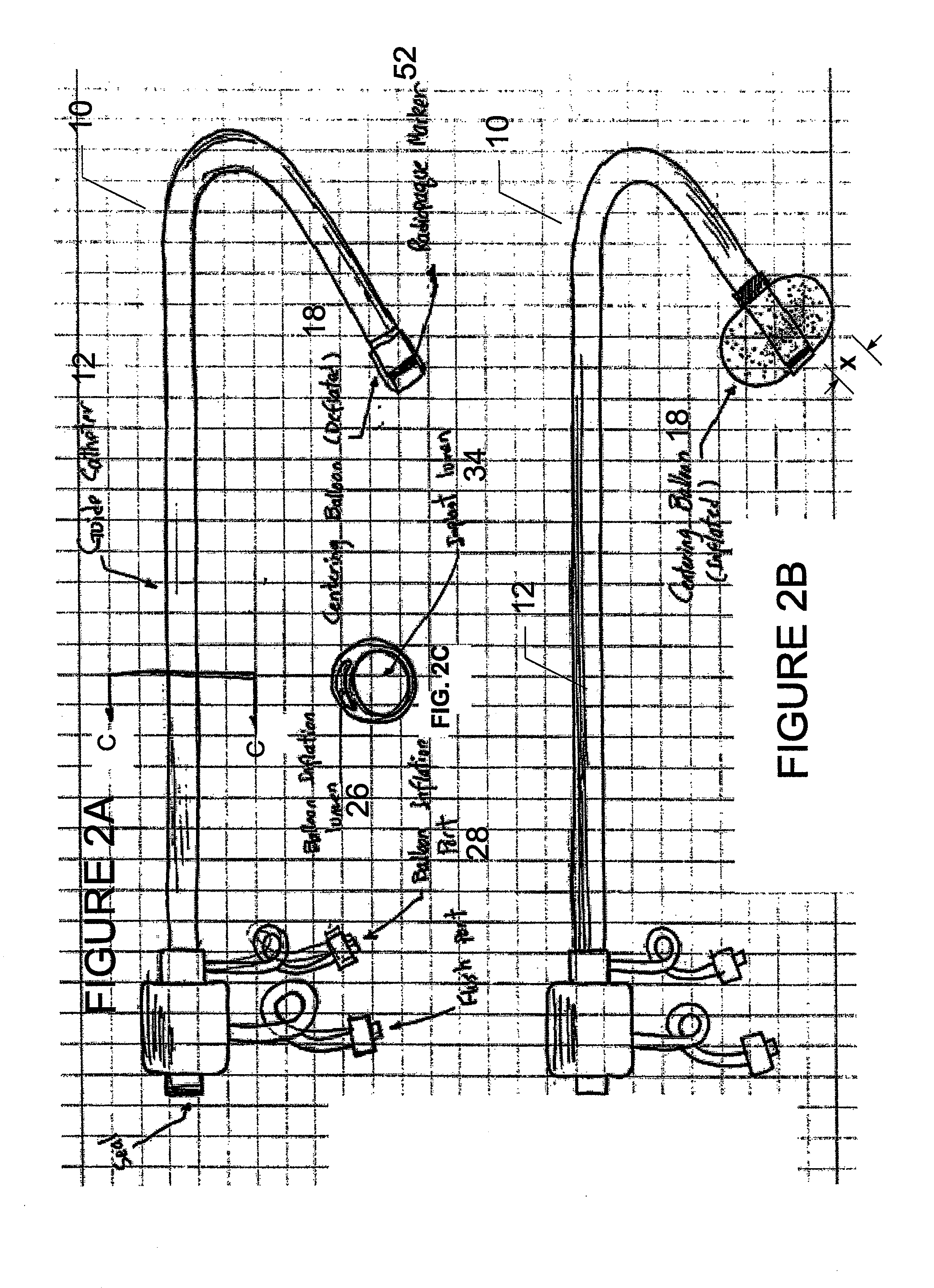
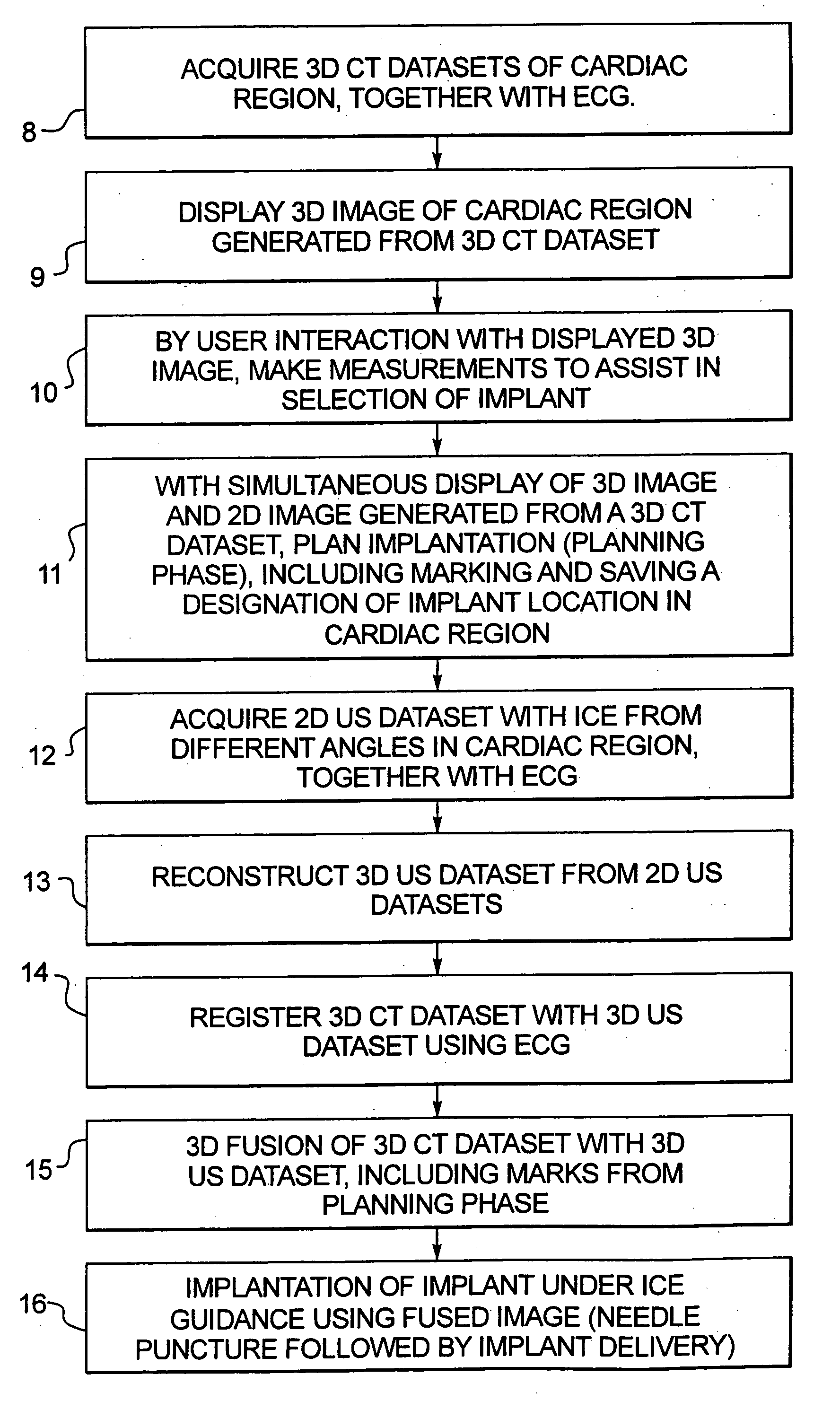
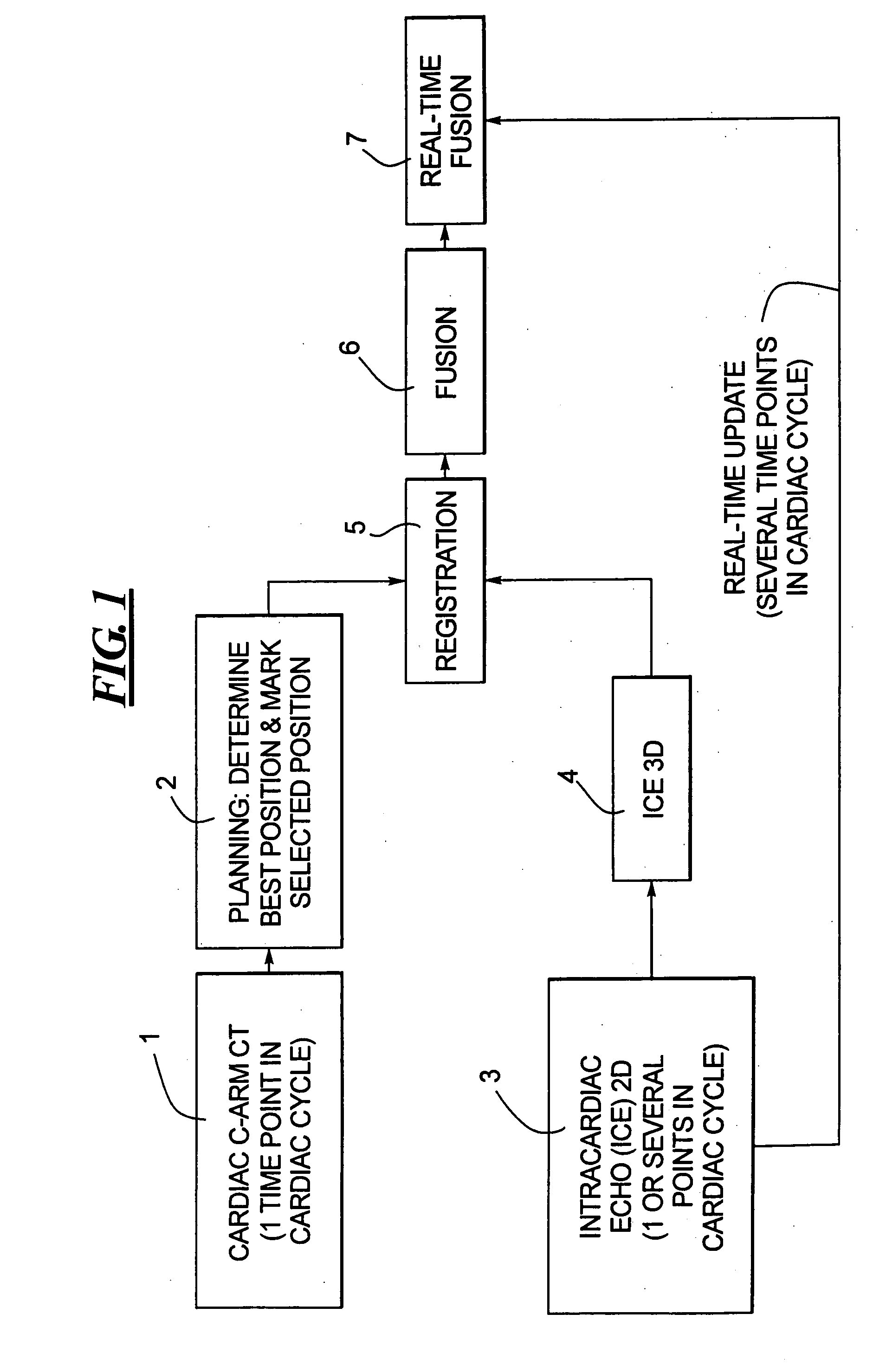
Method for implanting a cardiac implant with real-time ultrasound imaging guidance
InactiveUS20080146919A1Reduce disadvantagesUltrasonic/sonic/infrasonic diagnosticsCatheterUltrasound imaging2d ultrasound
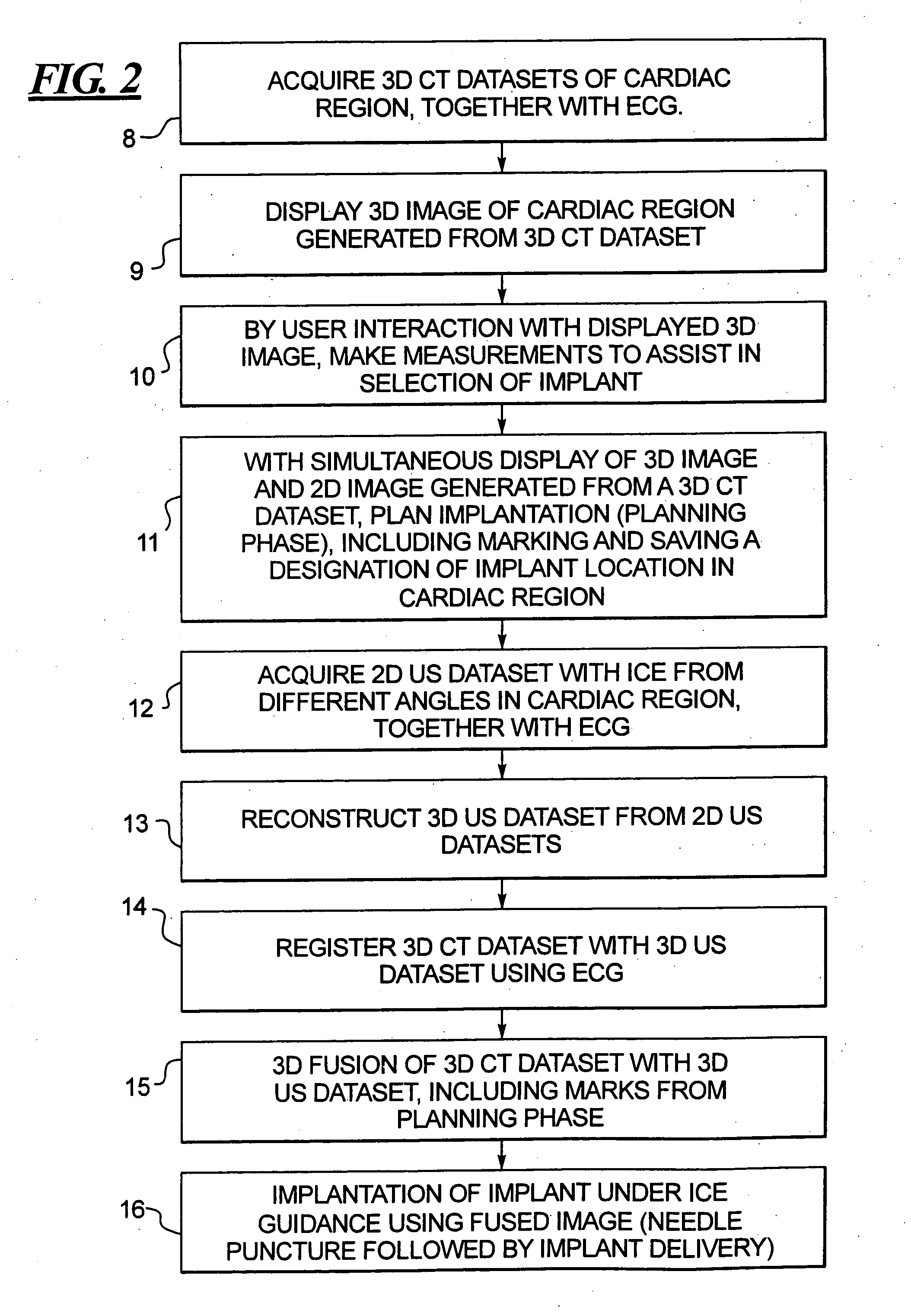
In a method for implanting a cardiac implant, a 3D CT dataset of a cardiac region of interest at which an implant is to be implanted, is displayed and the implantation procedure is planned, which includes the physician electronically marking a best implantation site in the displayed image. This marking is then included in the 3D CT dataset. A 3D ultrasound dataset of the region of interest is acquired, and is brought into registration with the 3D CT dataset that incorporates the marking, and a fused image is produced therefrom. The fused image is displayed during the implantation procedure, and is updated with multiple real-time 2D ultrasound images obtained using the catheter that is employed to deliver the implant to the implantation site.
Owner:SIEMENS AG
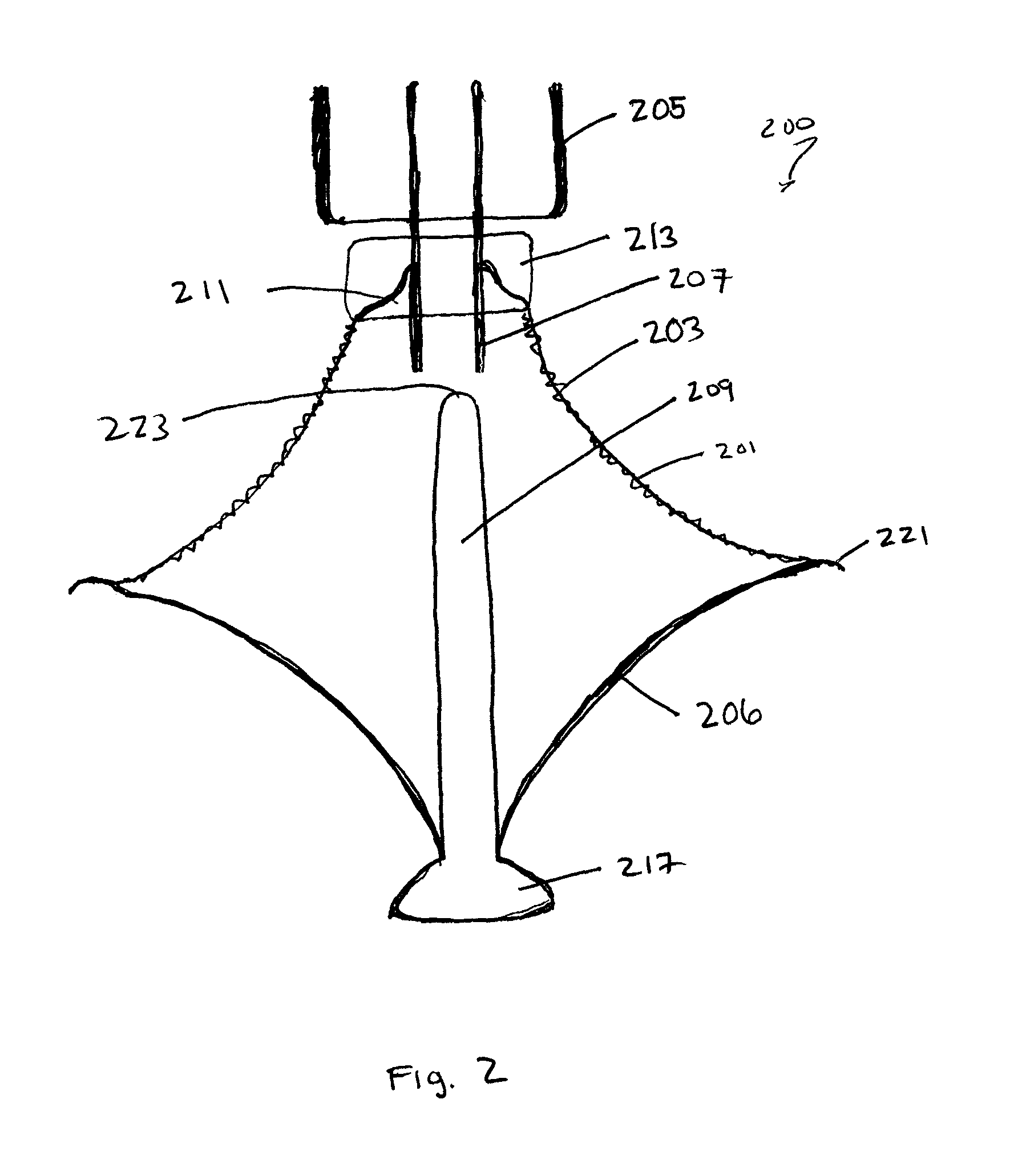
Multi-level cardiac implant
A heart valve prosthesis including a frame, the frame including a plurality of struts designed to extend from an upstream side of a natural heart valve to a downstream side of the natural heart valve, and a plurality of connectors attached to the plurality of struts, wherein the plurality of connectors are arranged as arcs connecting the struts, the arcs having two ends, each end attached to one of the struts, and a peak pointing from a center of the frame circumferentially outward and toward the upstream side of the frame, and the plurality of connectors are arranged as at least two rows, each row circumnavigating the center lumen of the frame. Related apparatus and methods are also described.
Owner:MITRASSIST MEDICAL
Retrievable cardiac devices
ActiveUS20100048987A1Shorten the lengthShortening of regionHeart valvesSurgical needlesCardiac deviceEndocrinology
Owner:EDWARDS LIFESCIENCES CORP
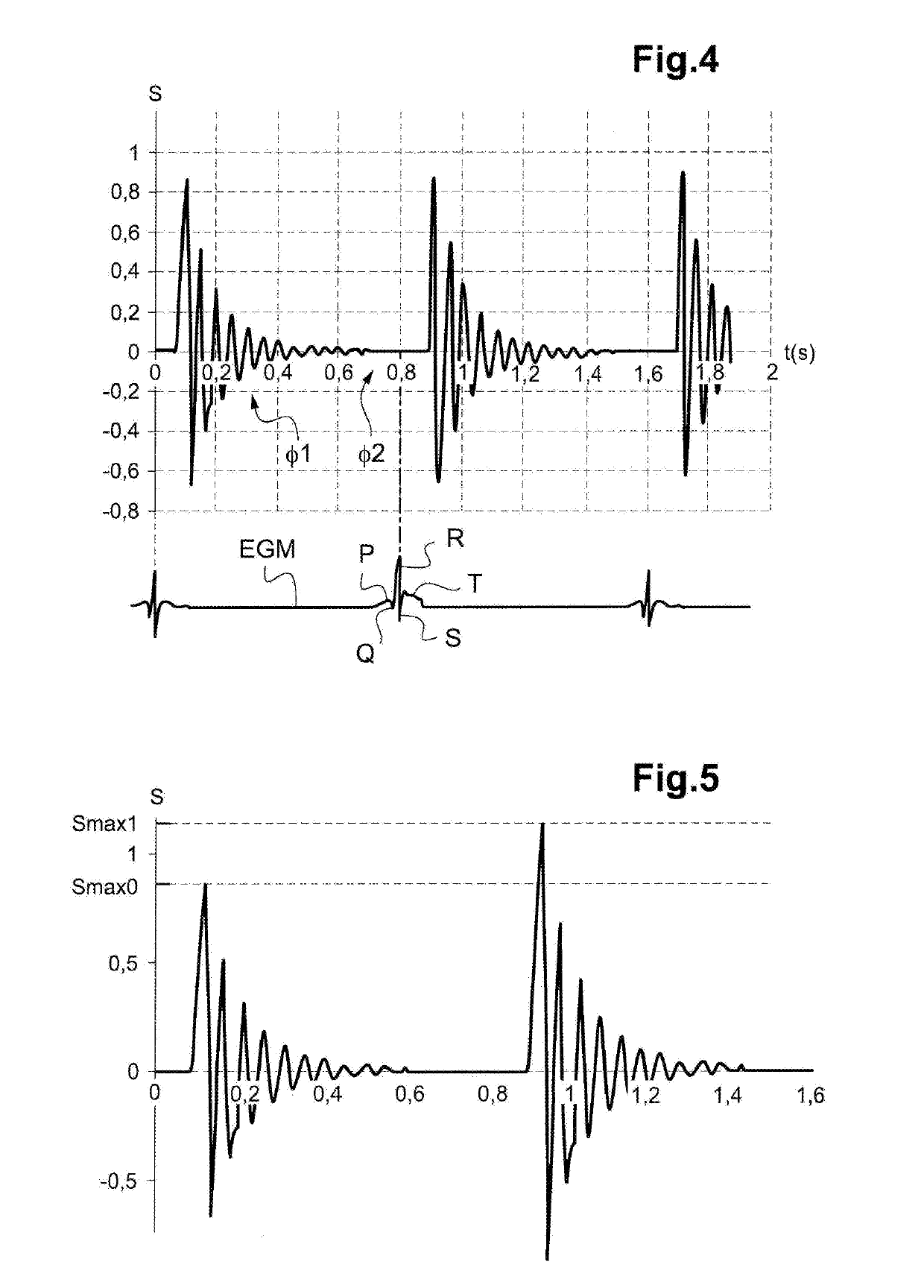
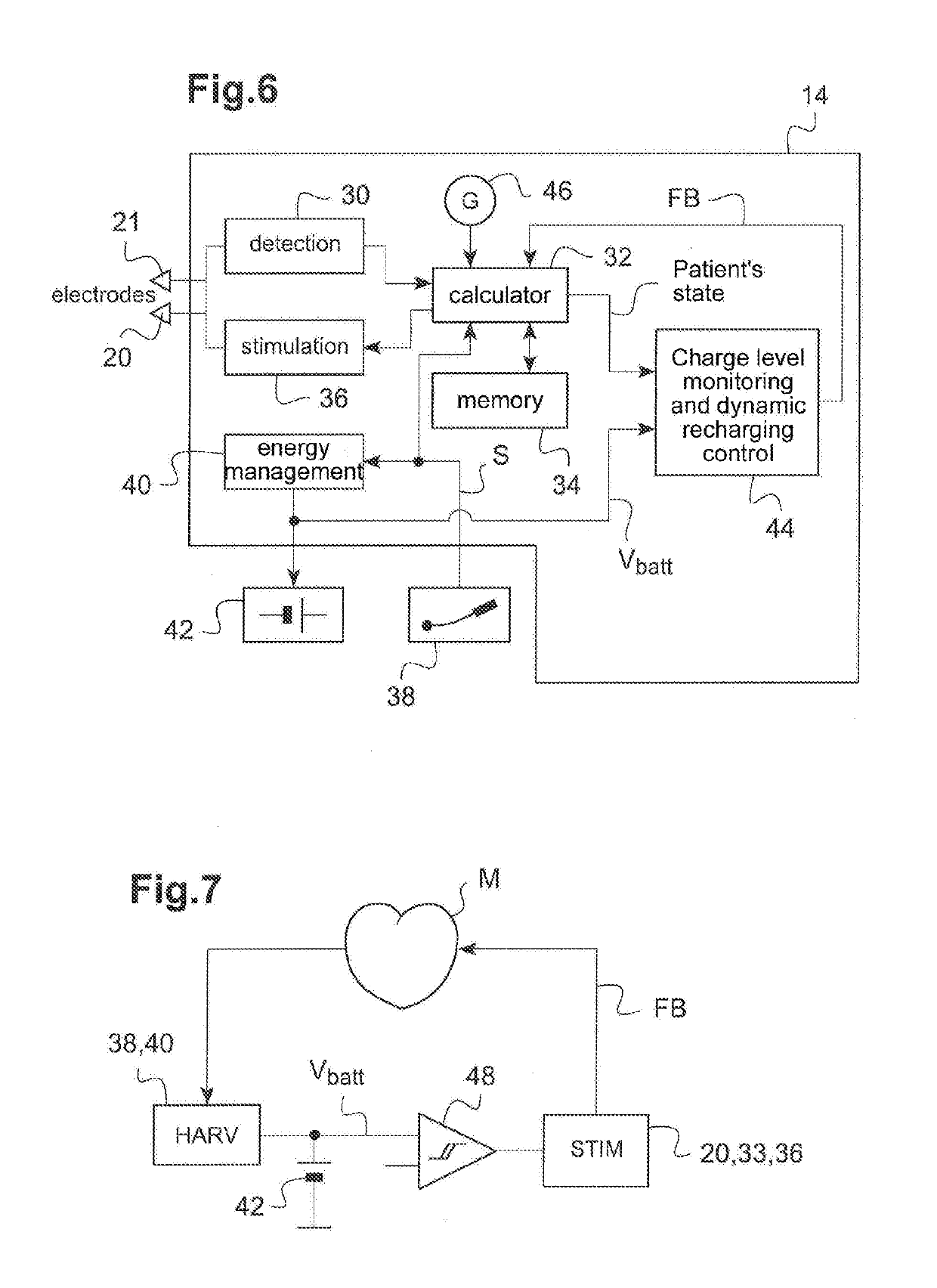
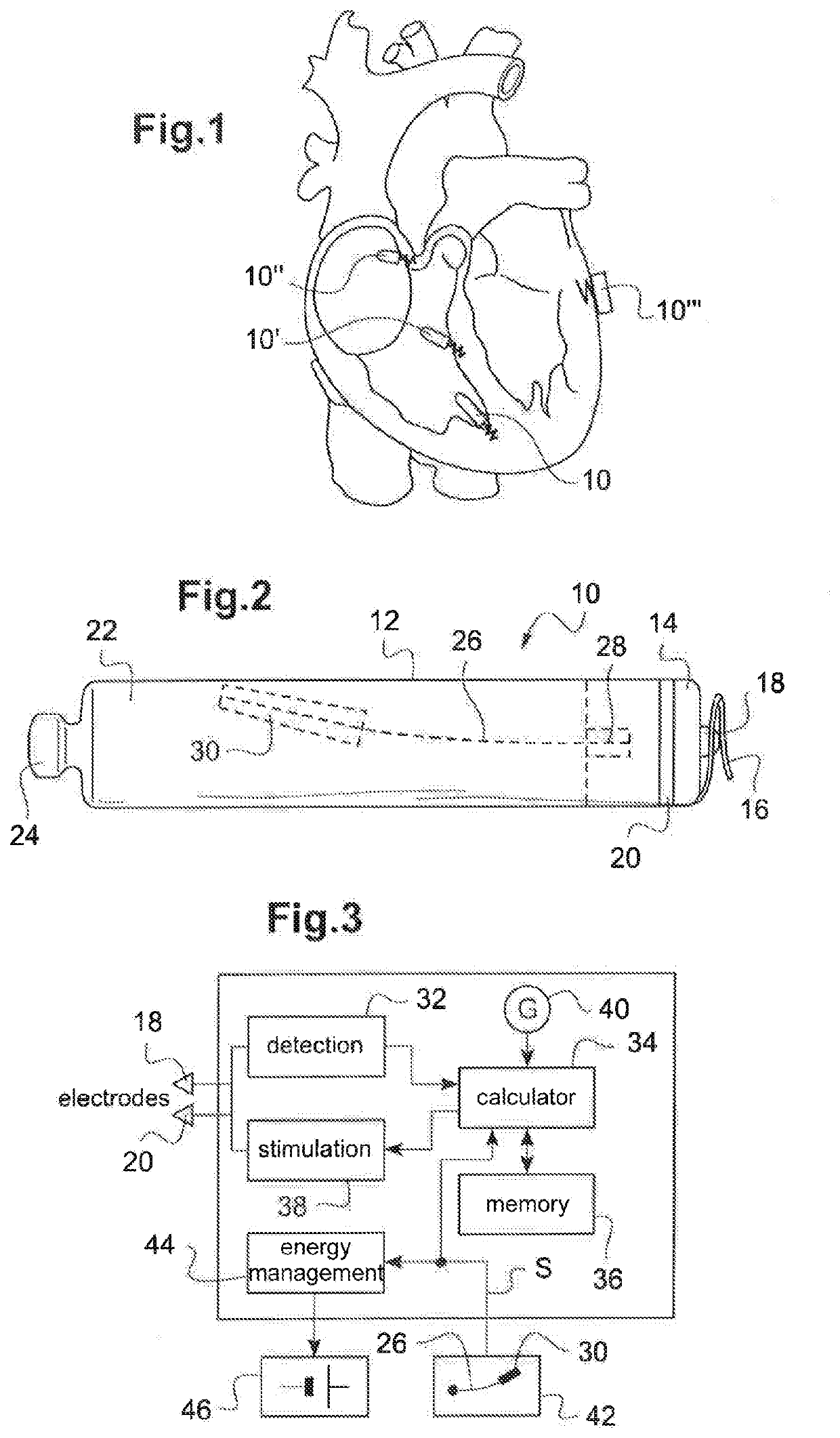
Autonomous cardiac implant of the leadless capsule type with energy harvester and controlled-charge energy storage buffer
ActiveUS20190091479A1ElectrocardiographyPiezoelectric/electrostriction/magnetostriction machinesEnergy harvesterMechanical energy
An energy harvester converts into electrical energy the external stresses applied to the implant at the heartbeat rhythm. This harvester comprises an inertial unit and a transducer delivering an electrical signal that is rectified and regulated for powering the implant and charging an energy storage component. The charge level of the energy storage component is compared with a lower threshold to detect an insufficient charge, and a dynamic charging control circuit modifies, as and whenever necessary, and if the current patient's state allows it, a stimulation parameter in a direction liable to increase in return the mean level of the mechanical energy that is produced and harvested.
Owner:CAIRDAC
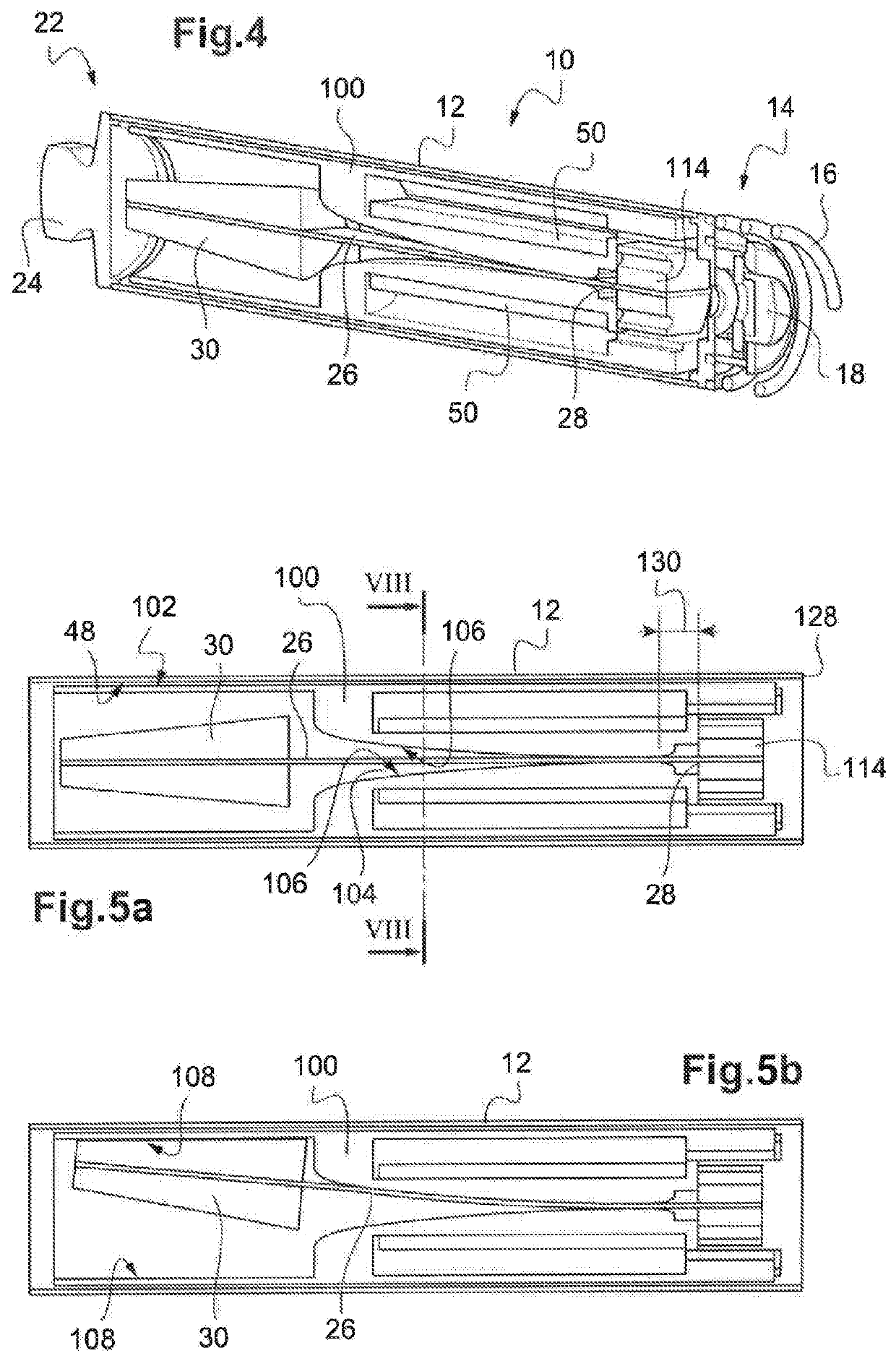
Autonomous cardiac implant of the leadless capsule type, including a piezoelectric beam energy harvester
ActiveUS20190381325A1Reduce free lengthElectrotherapyPiezoelectric/electrostriction/magnetostriction machinesInertial massEnergy harvester
The device includes an energy harvesting module with a pendular unit formed of an elastically deformable piezoelectric beam associated with an inertial mass. A multifunction part includes an axial through-recess with inner bearing surfaces opposite respective outer faces of the beam. These bearing surfaces having an increasing transverse spacing, such as, during an oscillation cycle, the beam comes into contact with one of the bearing surfaces, hence reducing the free length of the beam as the bending of the latter goes along. The multifunction part also allows rationalizing the manufacturing and the assembly of the capsule, with high-level integration of the inner components of the implant.
Owner:CAIRDAC
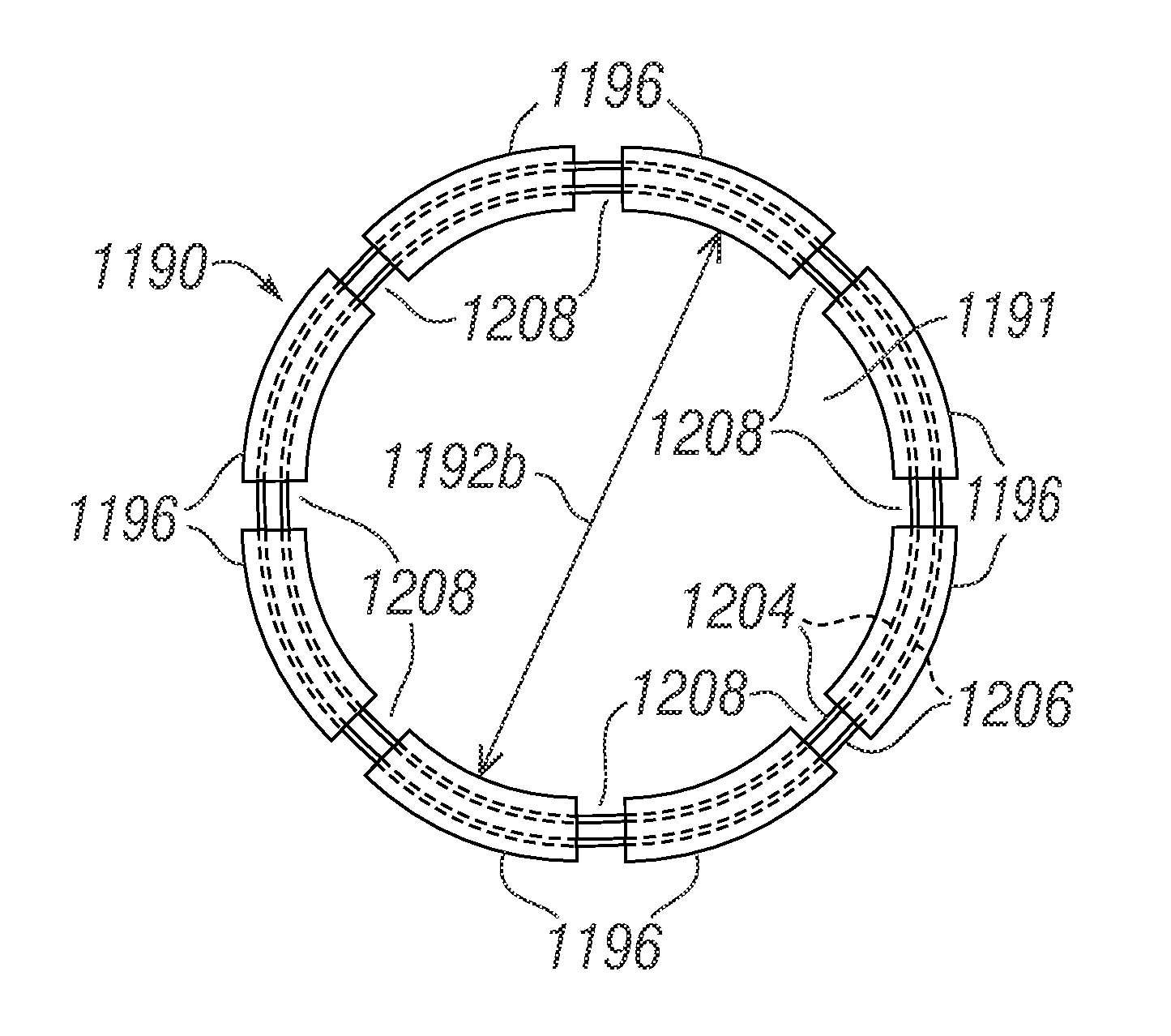
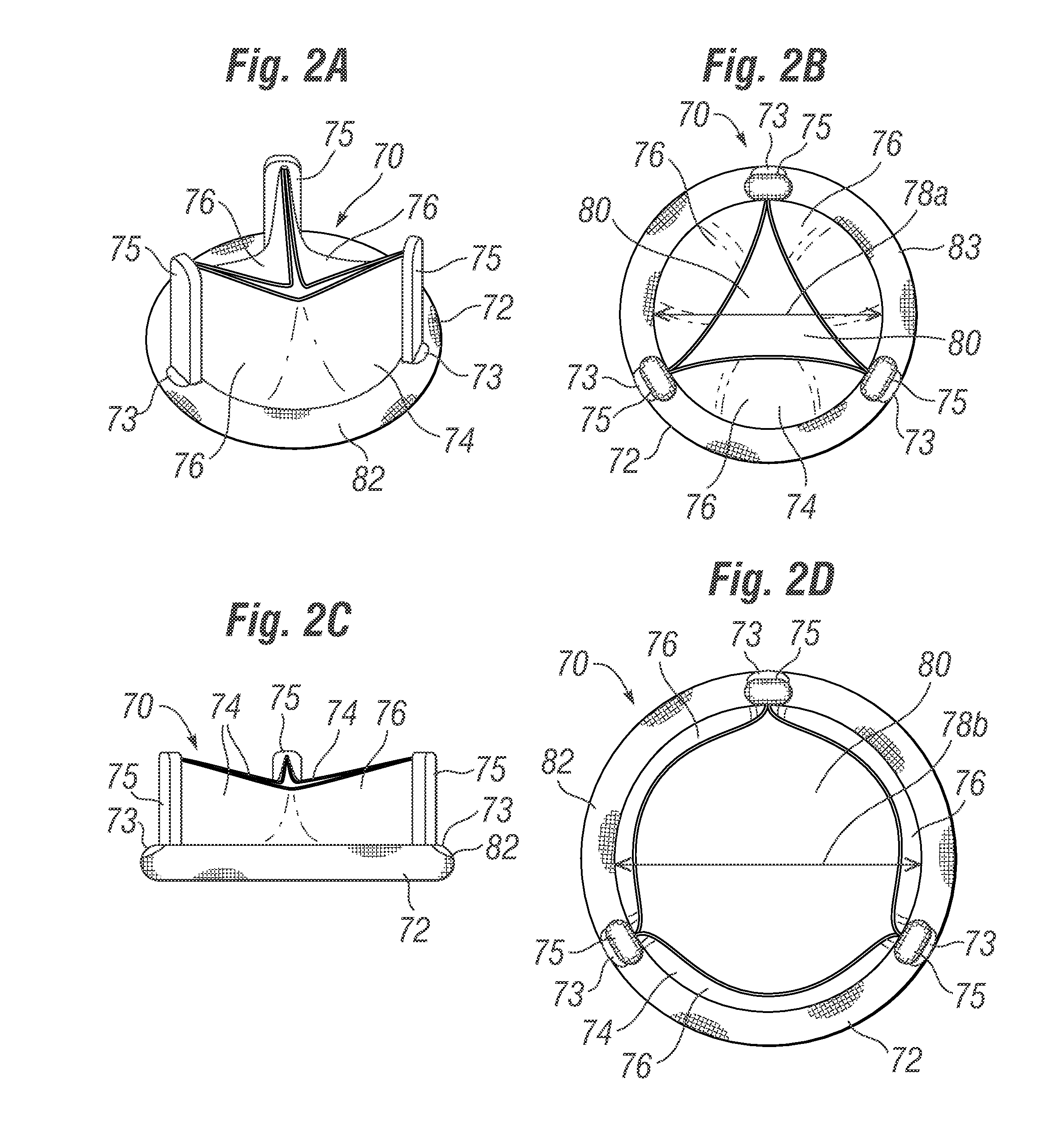
Collapsible cardiac implant and deployment system and methods
A collapsible device, such as an annuloplasty ring or prosthetic heart valve, is configured to be collapsed prior to being introduced into a patient via minimally-invasive access points such as port holes or intercostal incisions. A holder is configured to hold the collapsible device, and to selectively collapse the device for introduction into the patient and then re-enlarge the device at the desired deployment site. Collapsible devices include devices that can hingedly fold about hinge lines, and devices that can elongate to form substantially spiral forms with reduced diameters.
Owner:EDWARDS LIFESCIENCES CORP
Knotless suture fastener installation system
A knotless suture fastener installation system for securing medical devices such as cardiac implants. The knotless suture fasteners may be spring-biased so as to grip onto sutures passed therethrough. The system includes a fastener deployment tool with a proximal handle and a distal shaft to which a fastener cartridge attaches. A plurality of disposable cartridges are sequentially attached to the end of the deployment tool and used to secure the medical implant one fastener at a time. The deployment tool may also cut the sutures being fastened.
Owner:EDWARDS LIFESCIENCES CORP
Ventricular volume reduction
Devices and systems including implants (which may be removable) and methods of using them for reducing ventricular volume. The implants described herein are cardiac implants that may be inserted into a patient's heart, particularly the left ventricle. The implant may support the heart wall, or may be secured to the heart wall. The implants are typically ventricular partitioning device for partitioning the ventricle into productive and non-productive regions in order to reduce the ventricular volume.
Owner:EDWARDS LIFESCIENCES CORP
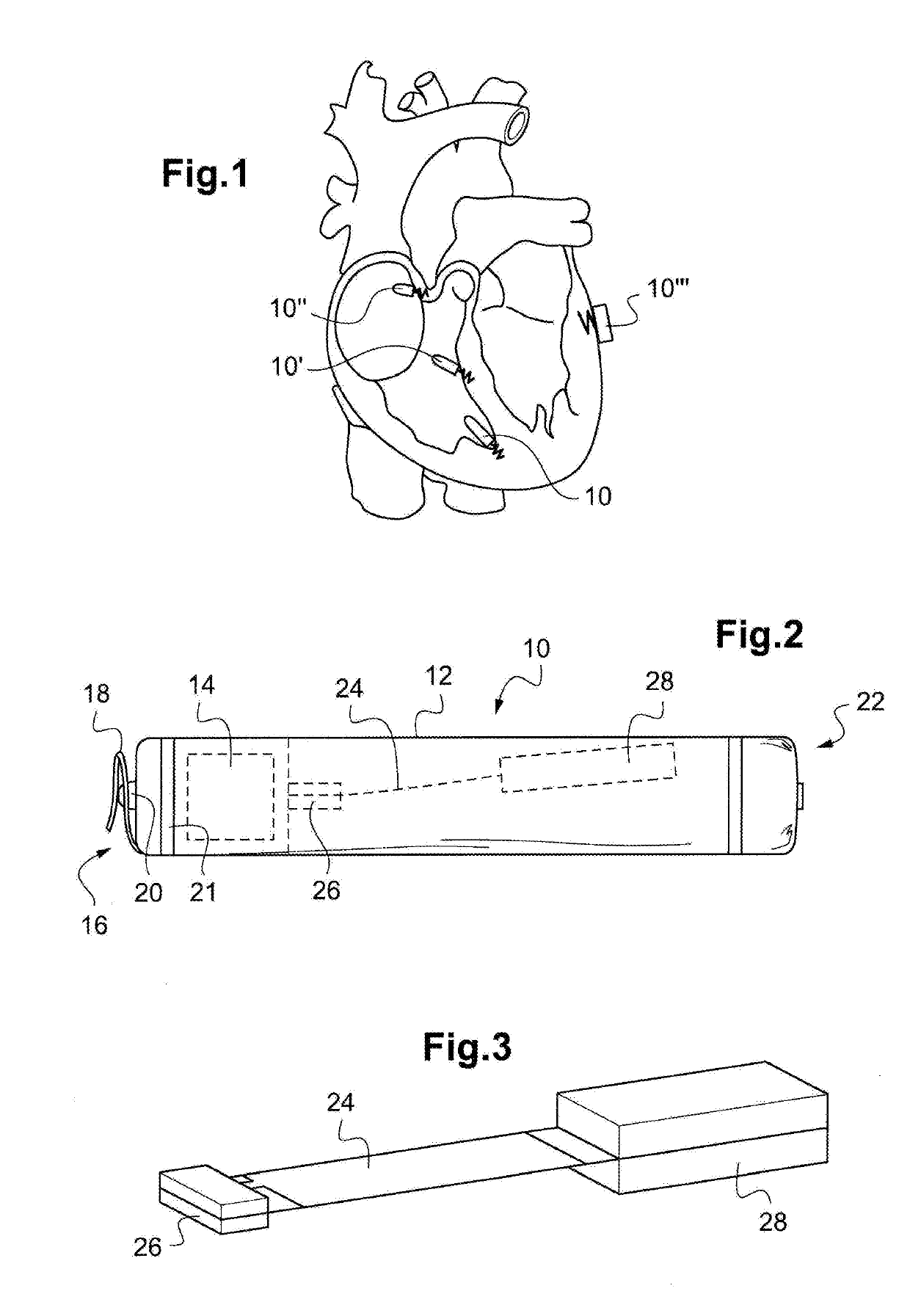
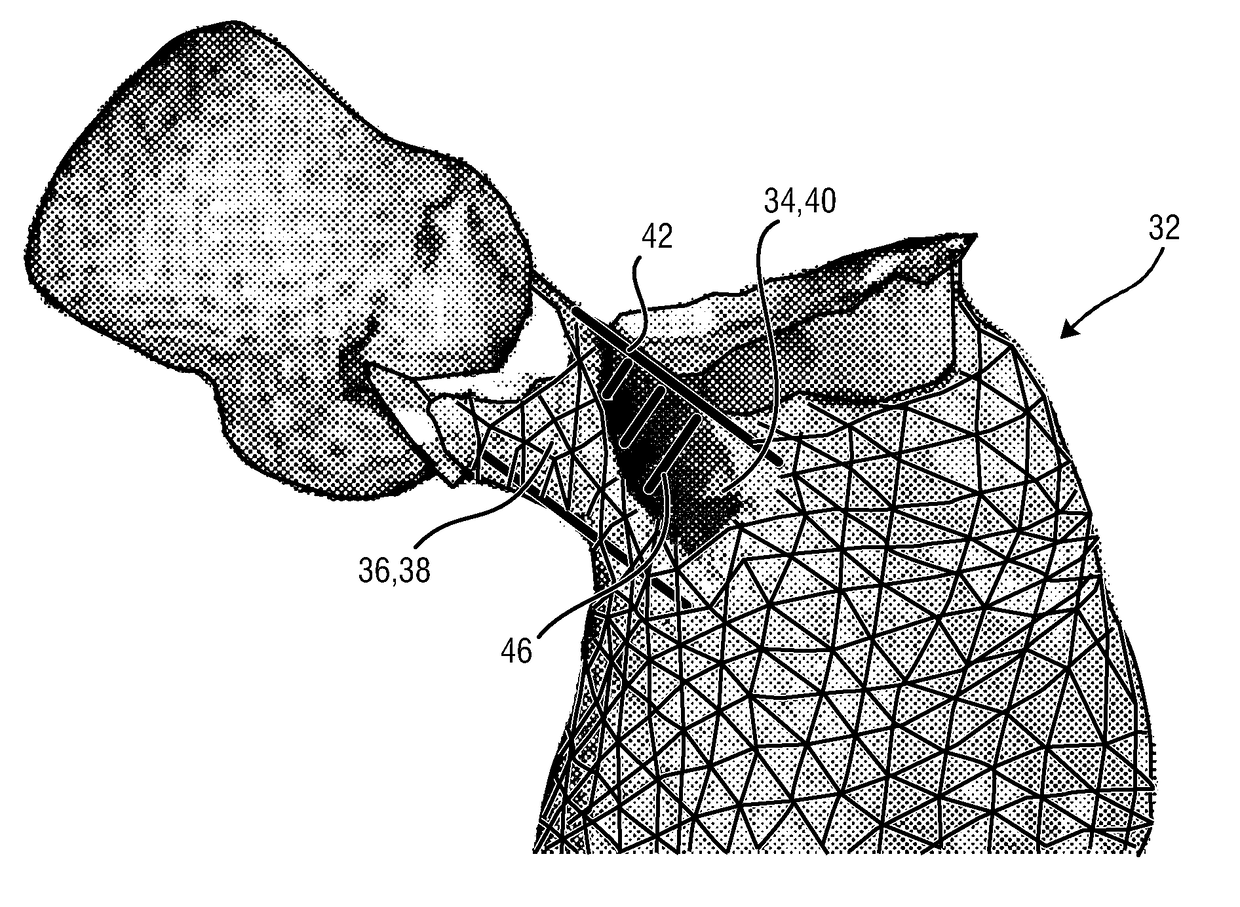
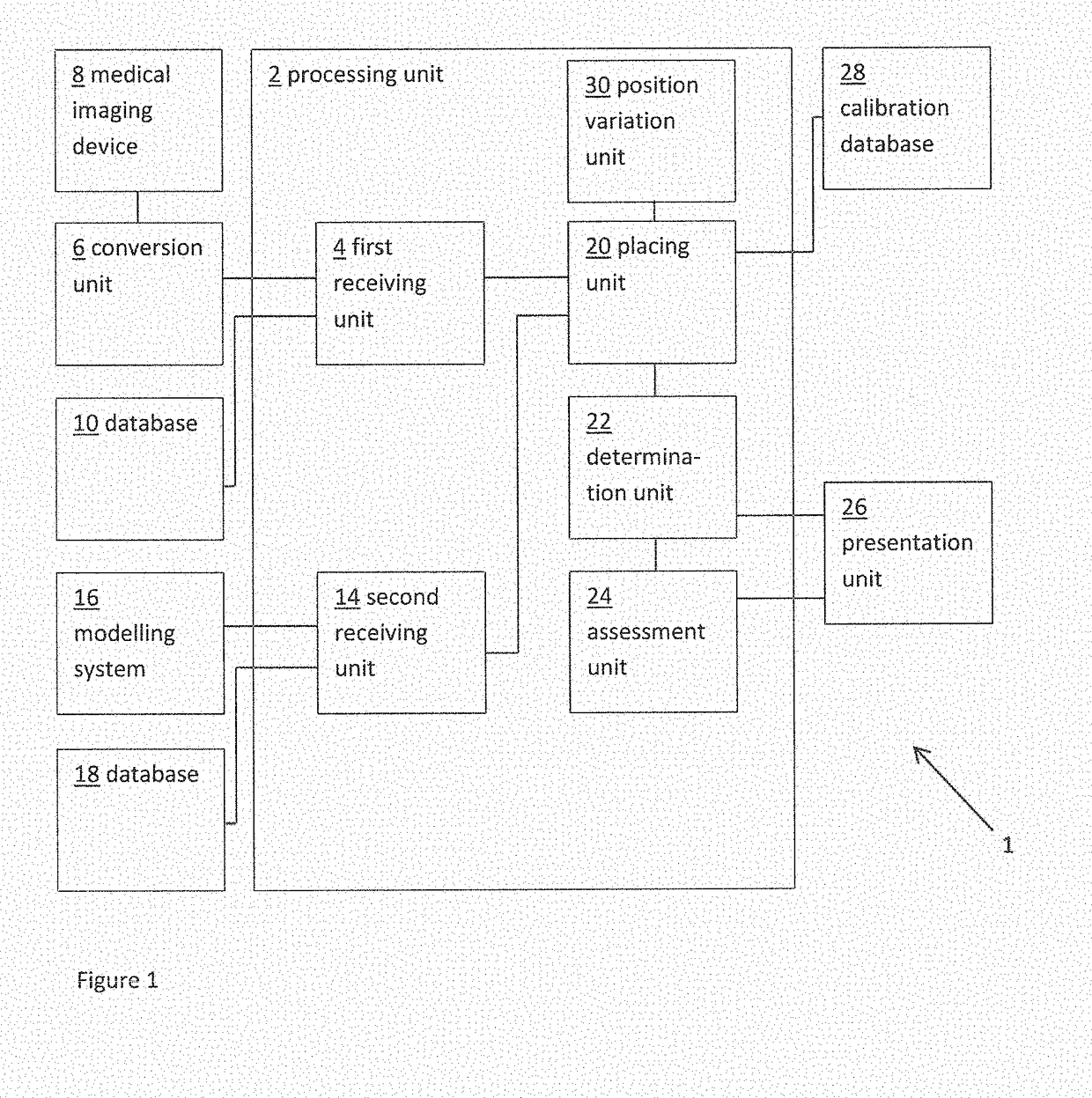
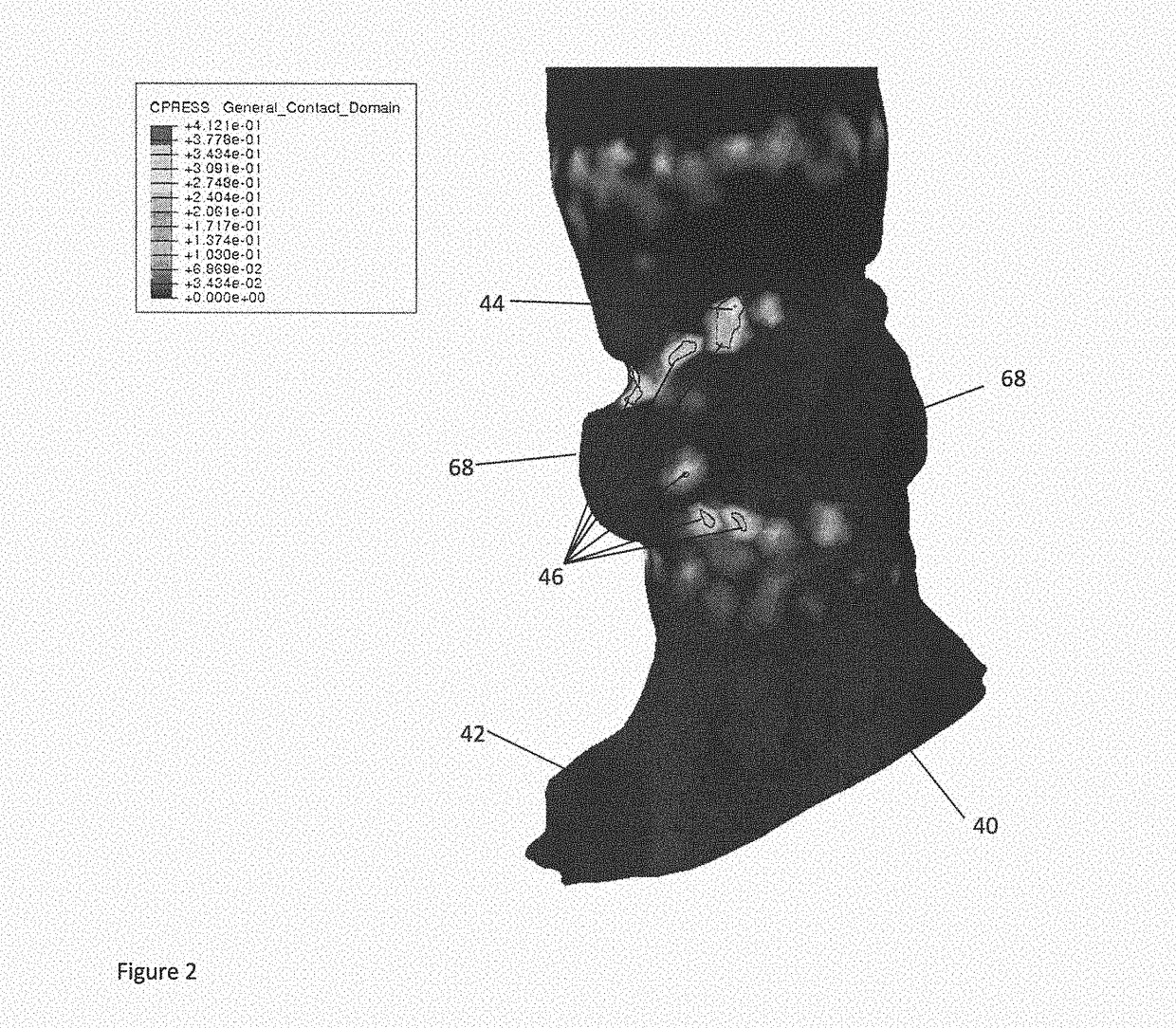
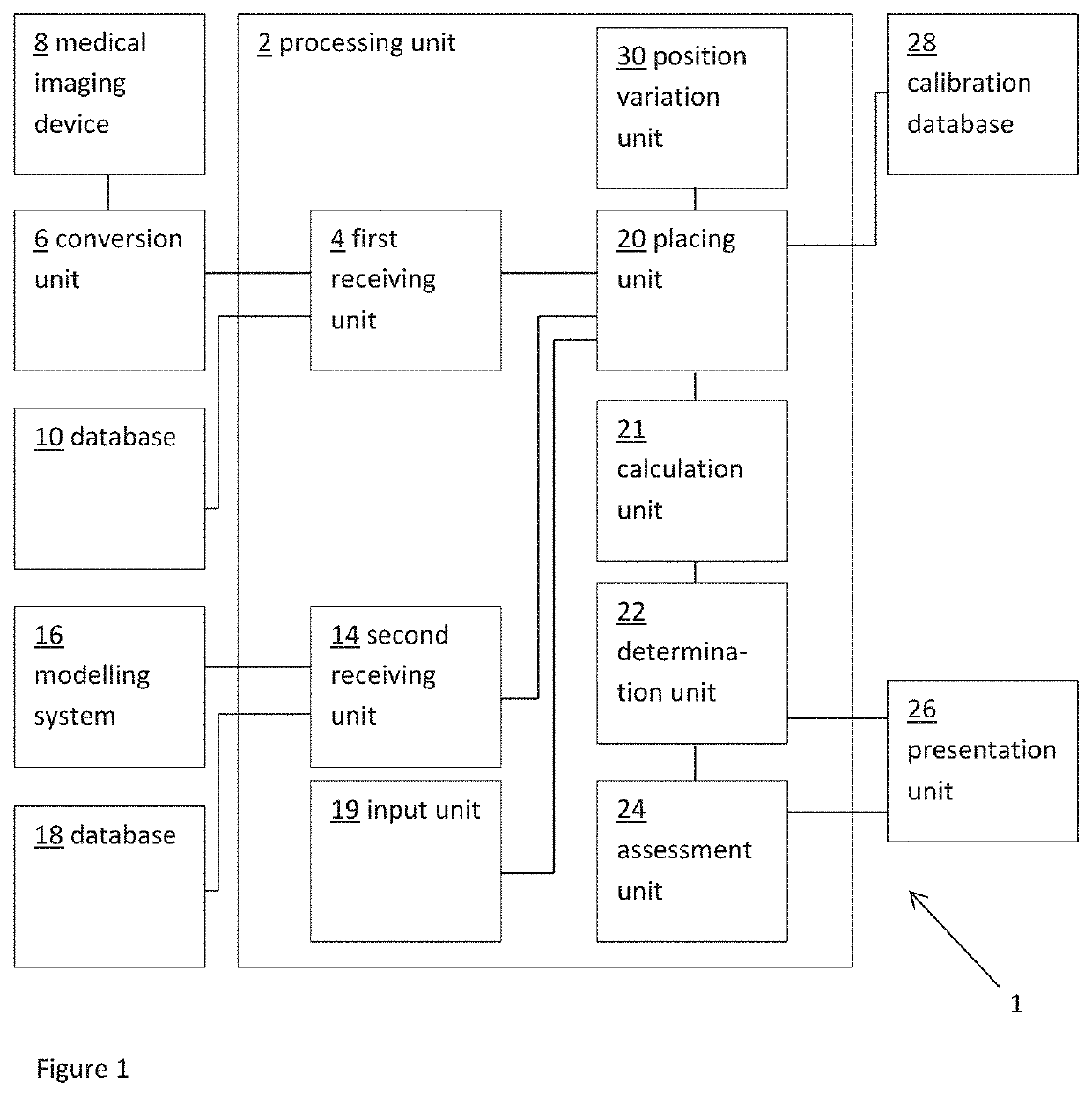
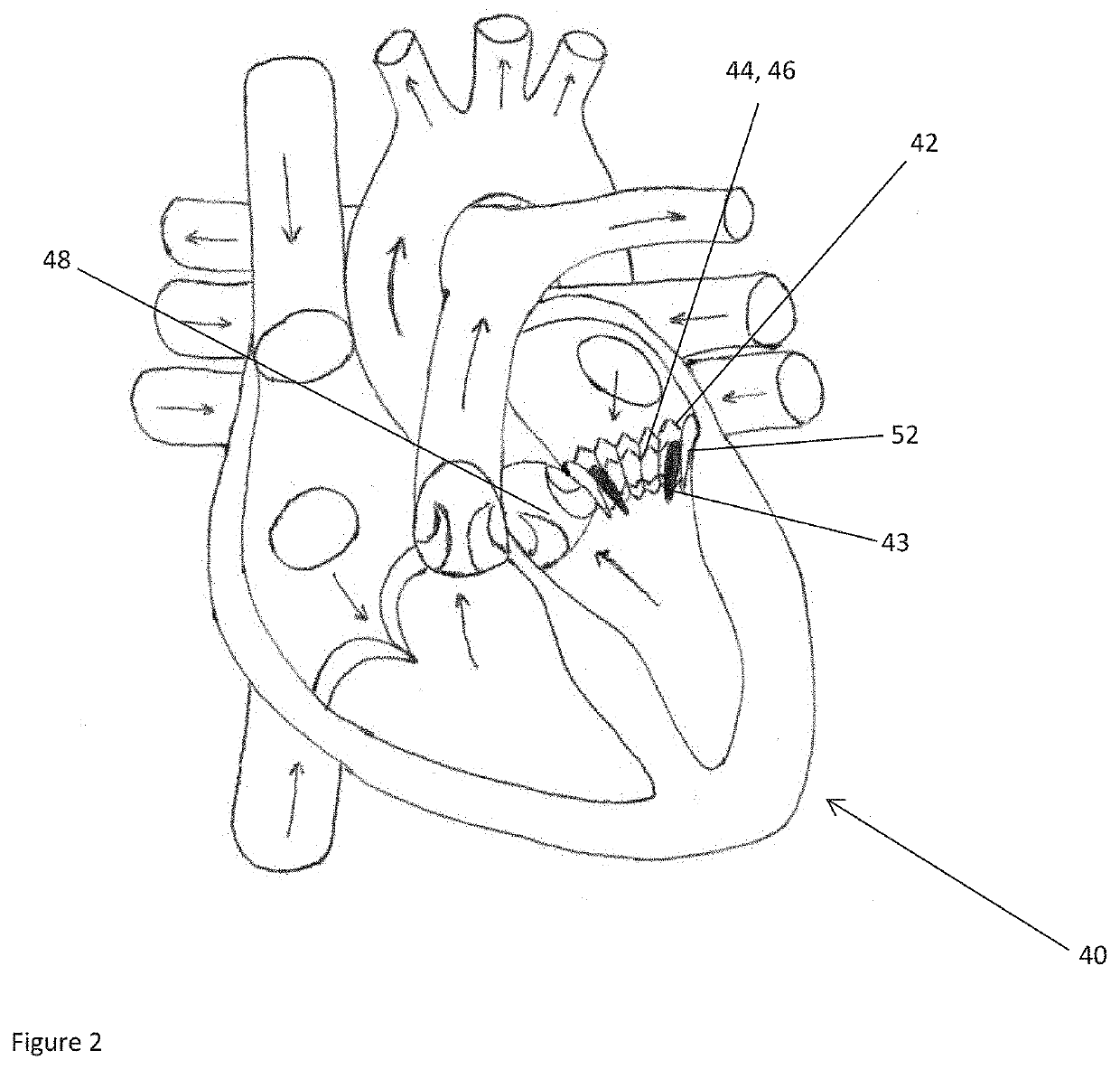
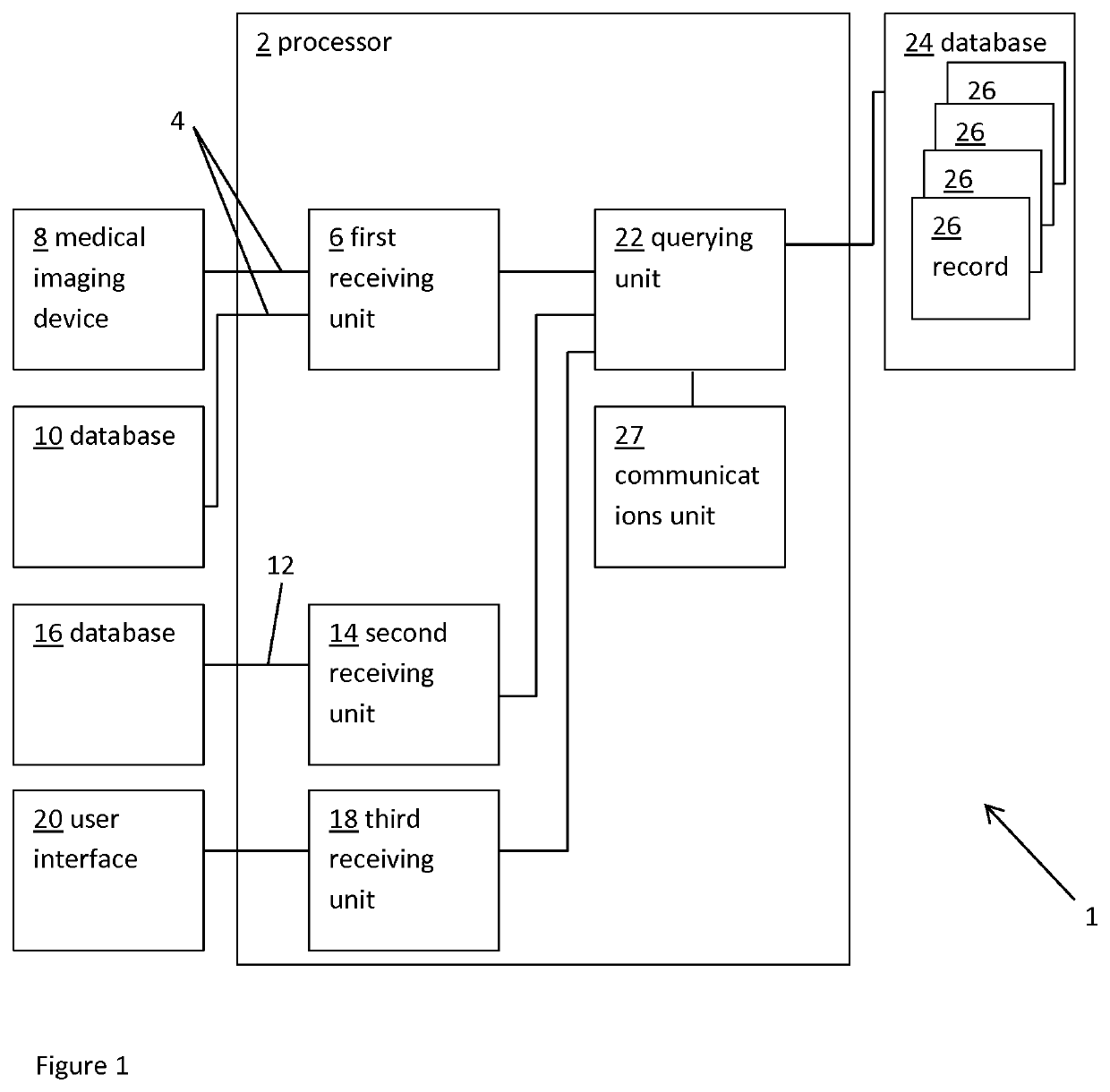
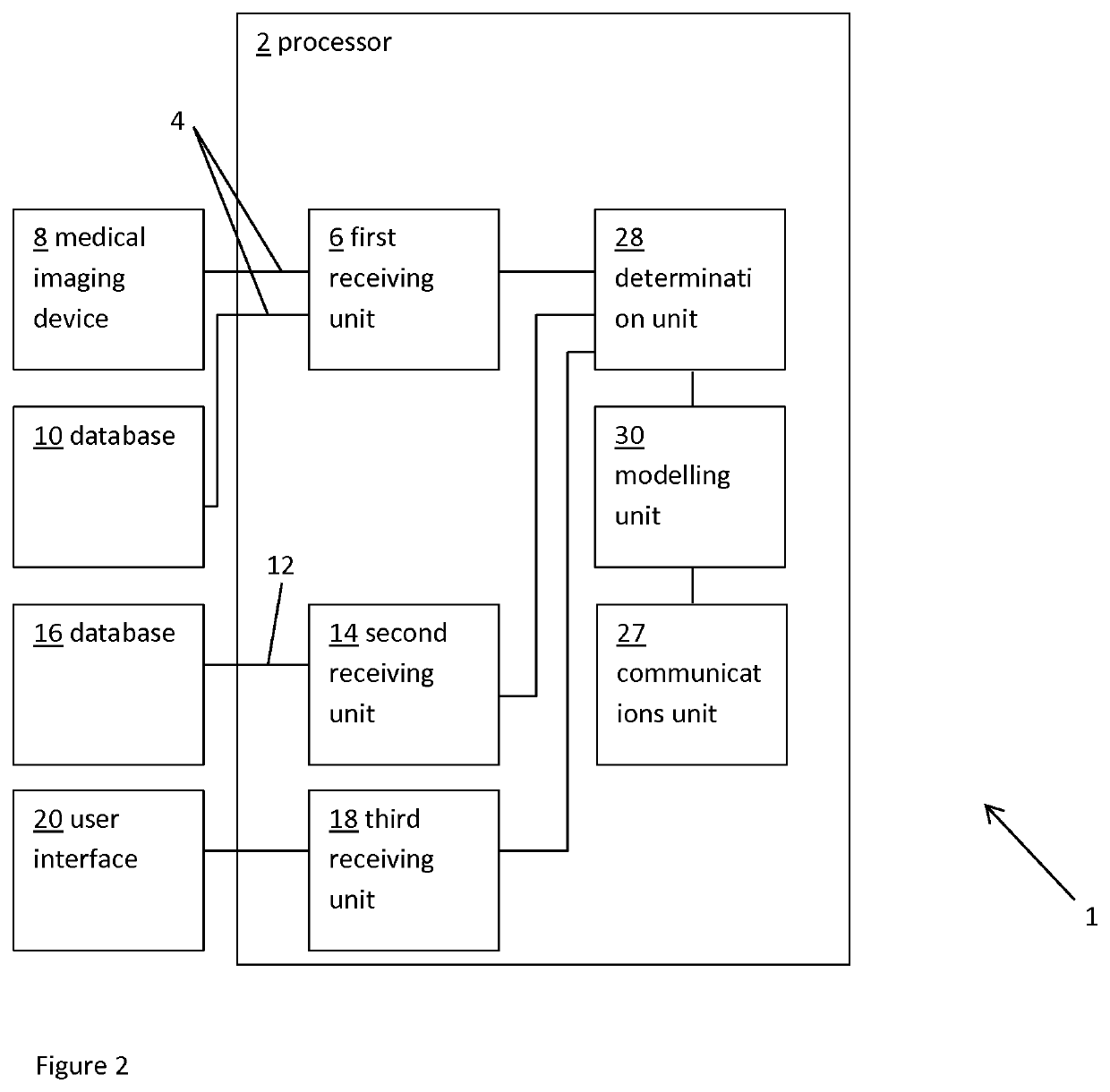
Planning an implantation of a cardiac implant
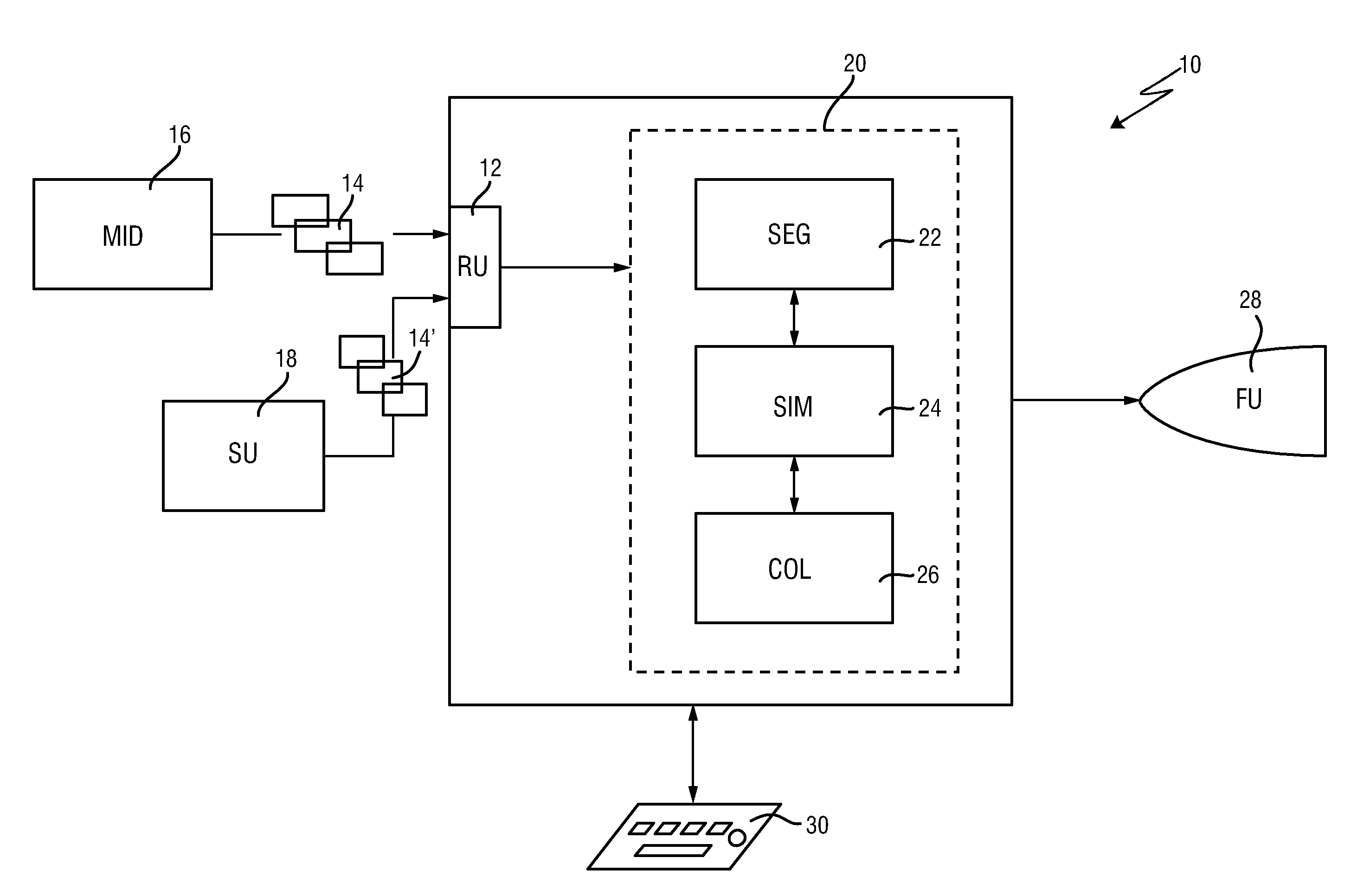
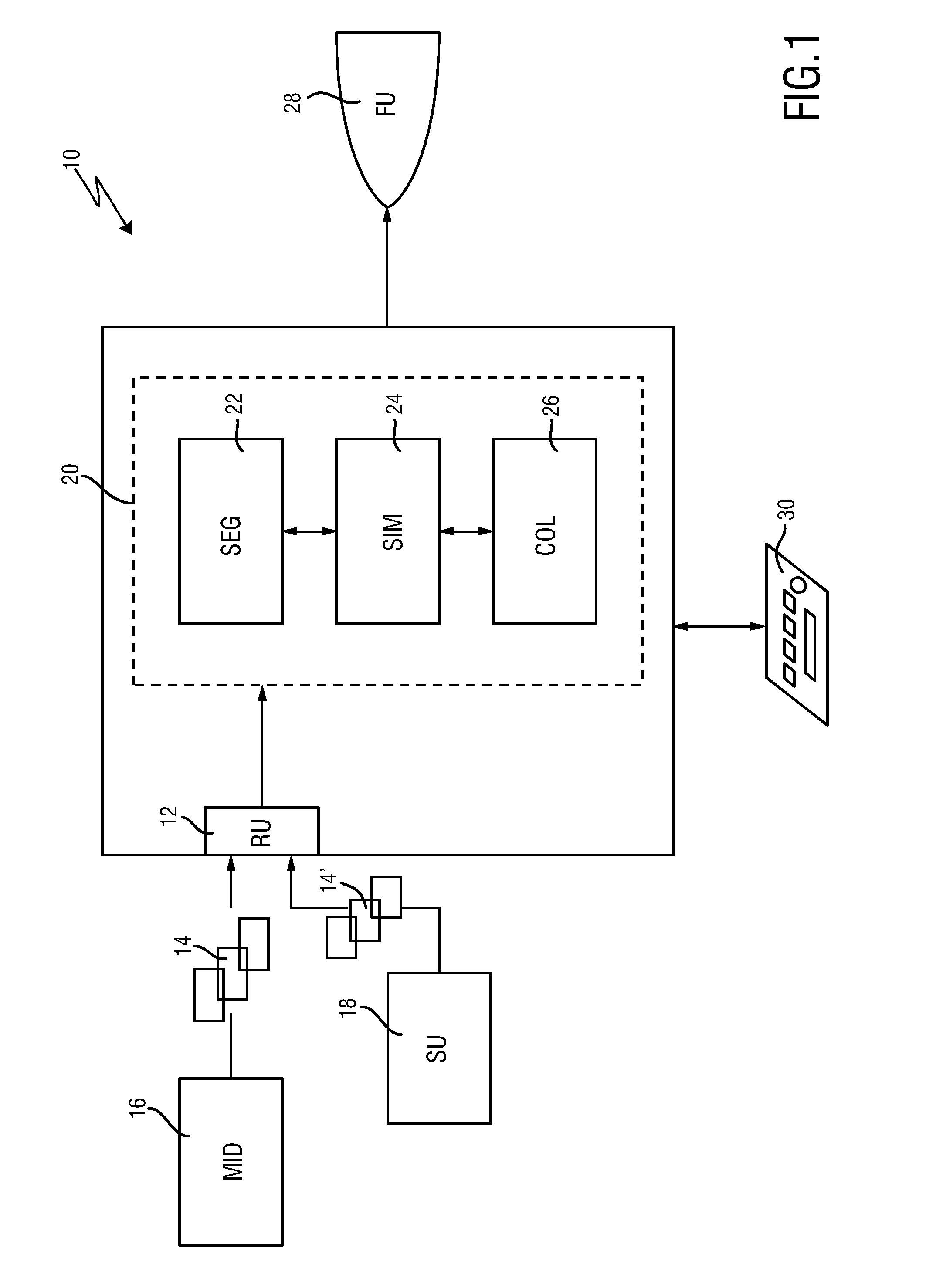
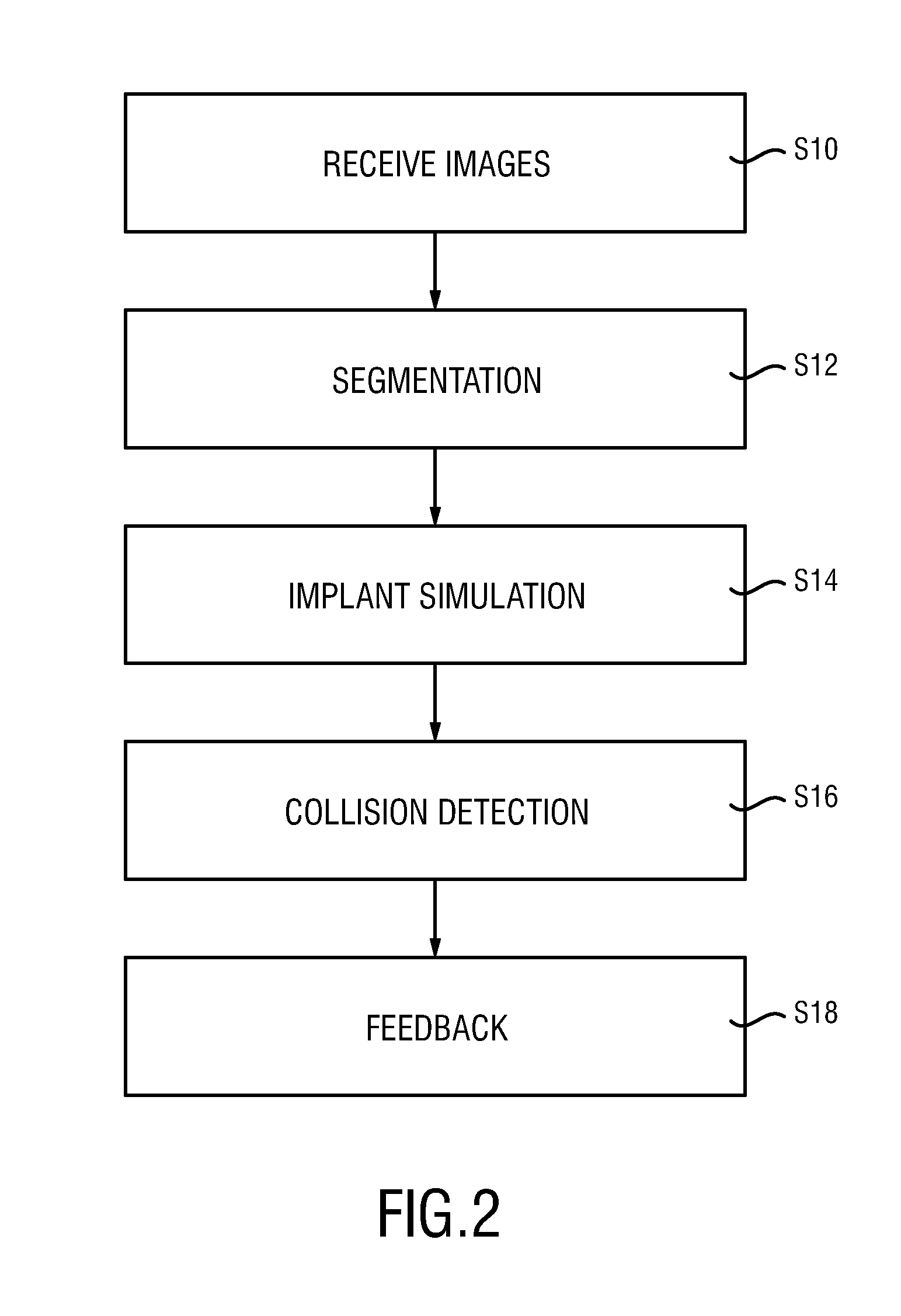
ActiveUS20160128786A1Accurately determineImage enhancementImage analysisCardiac cycleMedical imaging
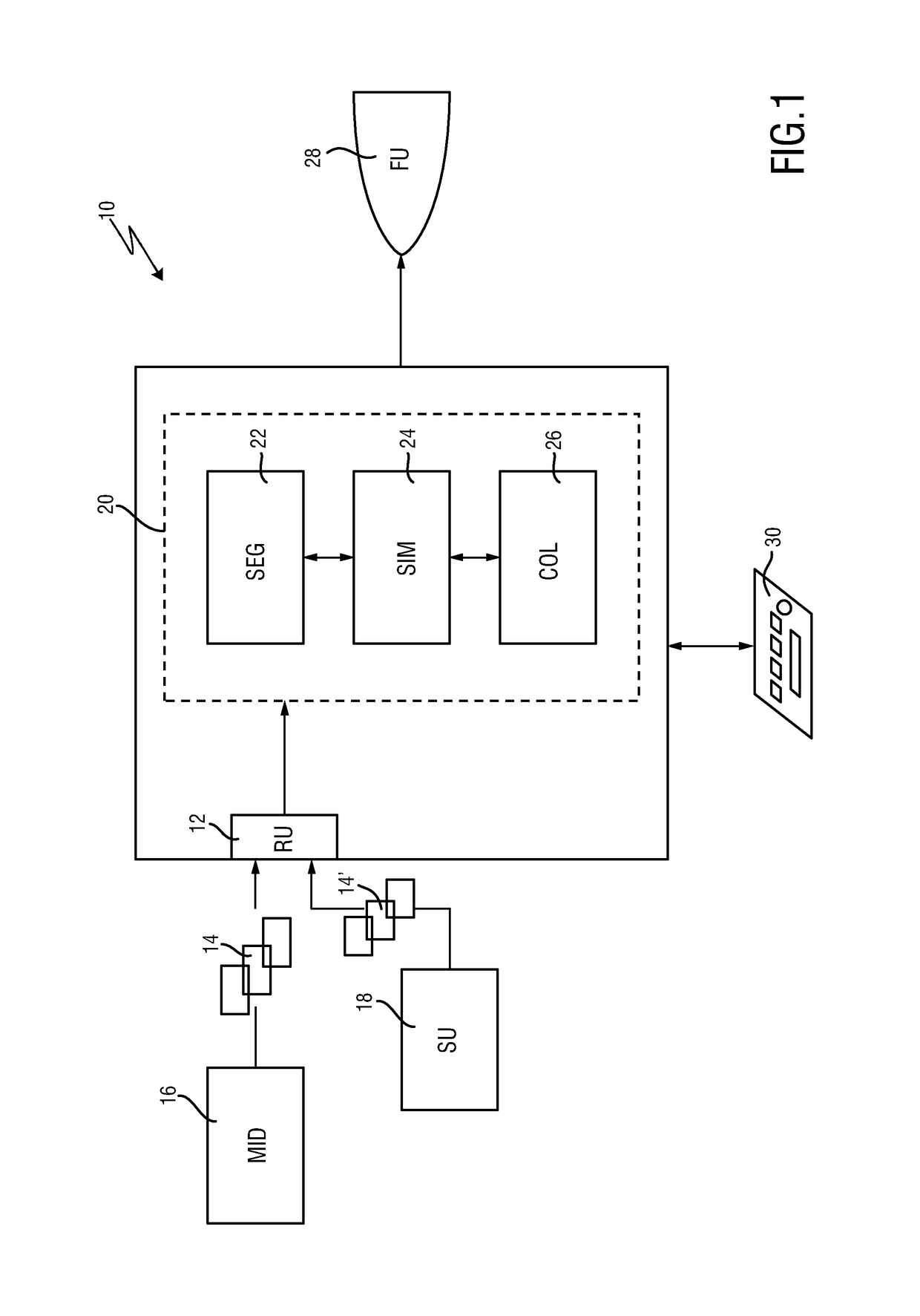
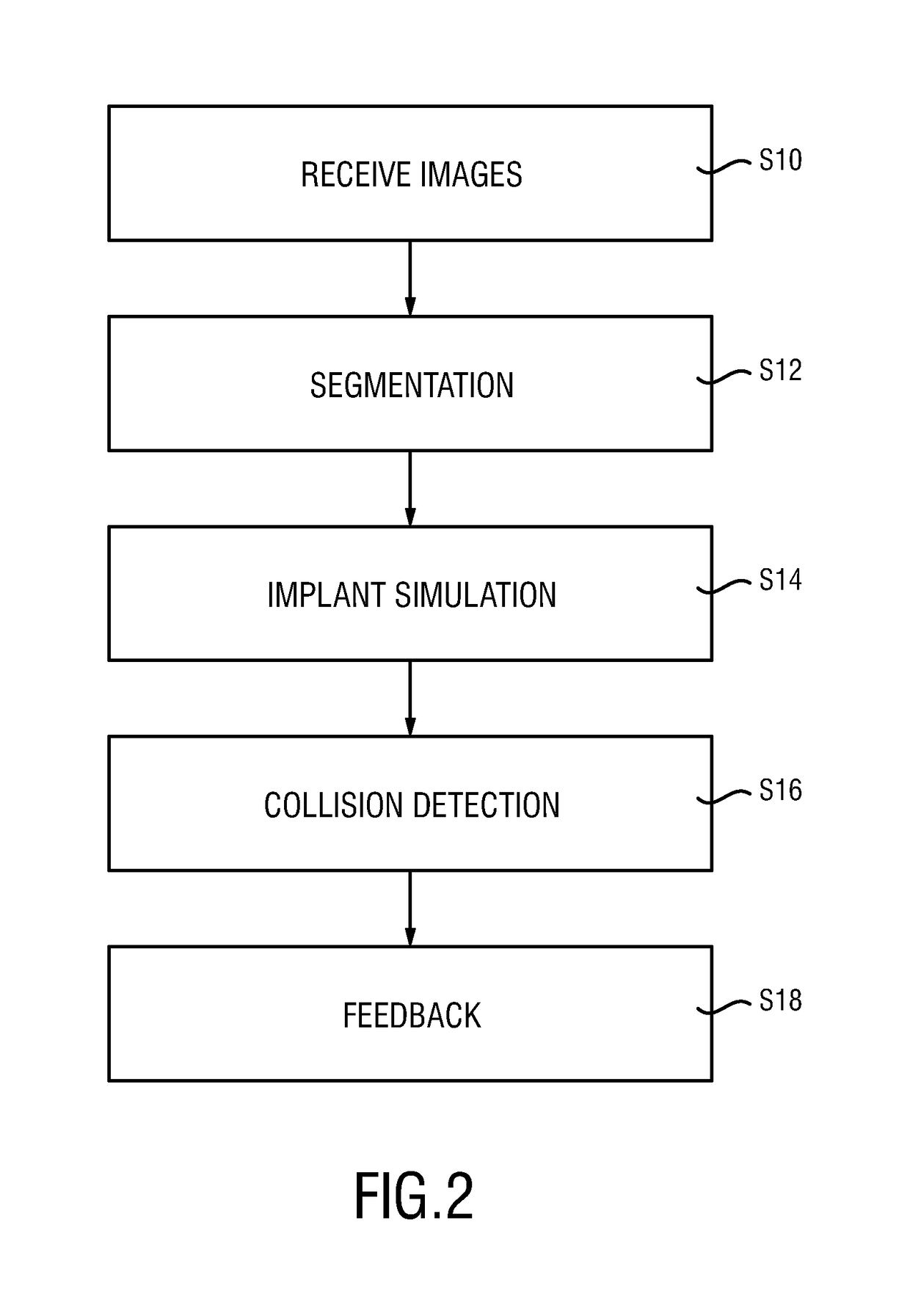
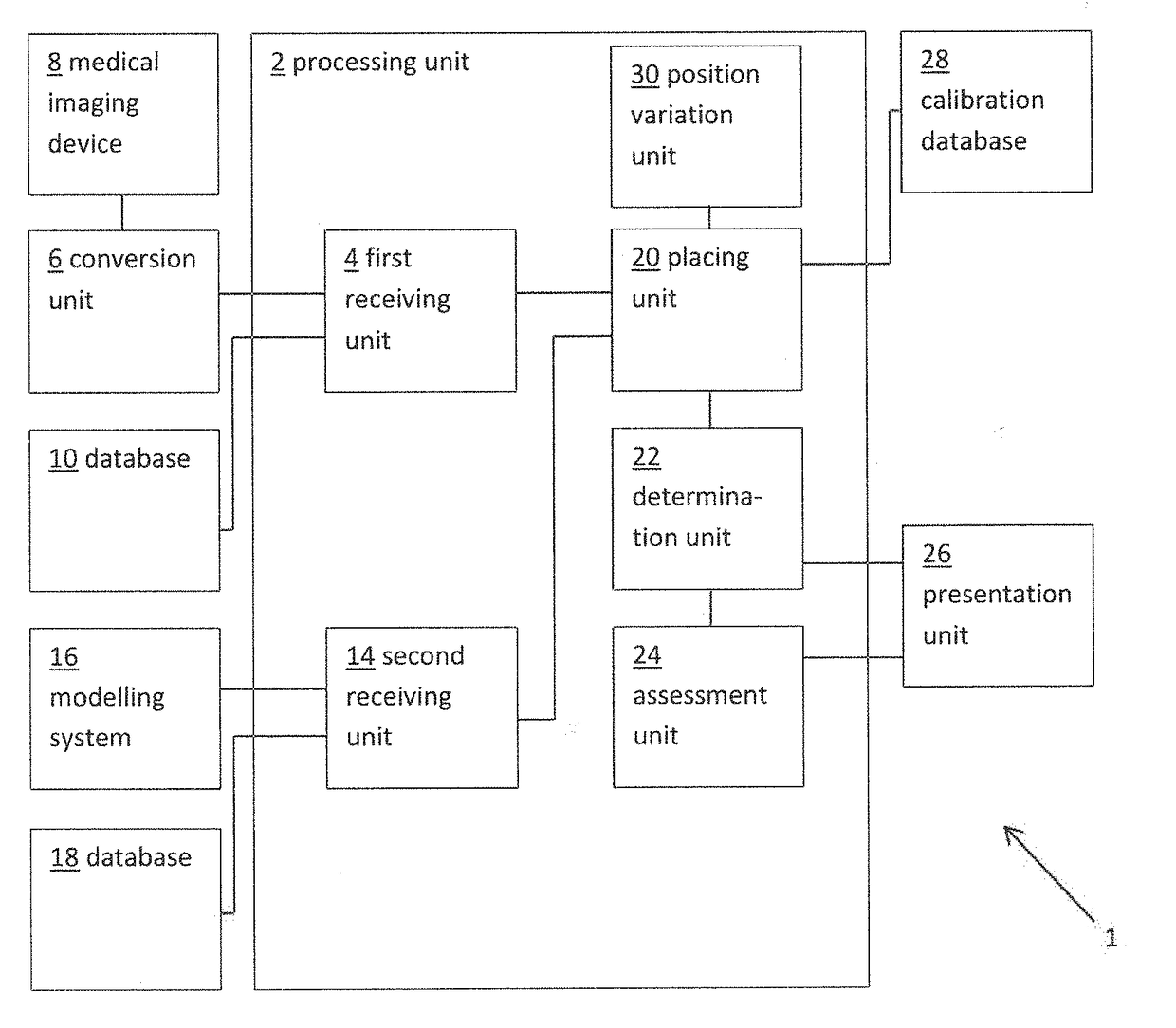
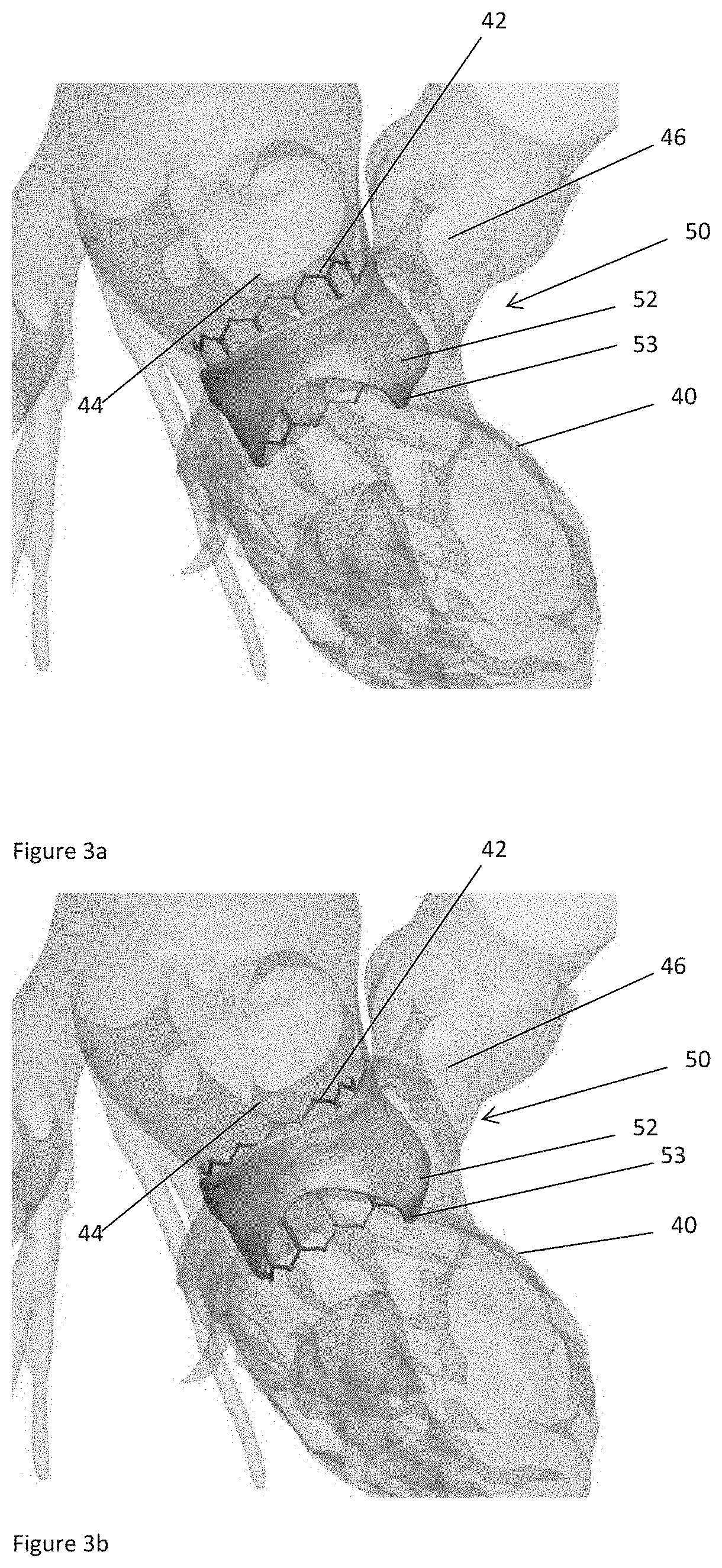
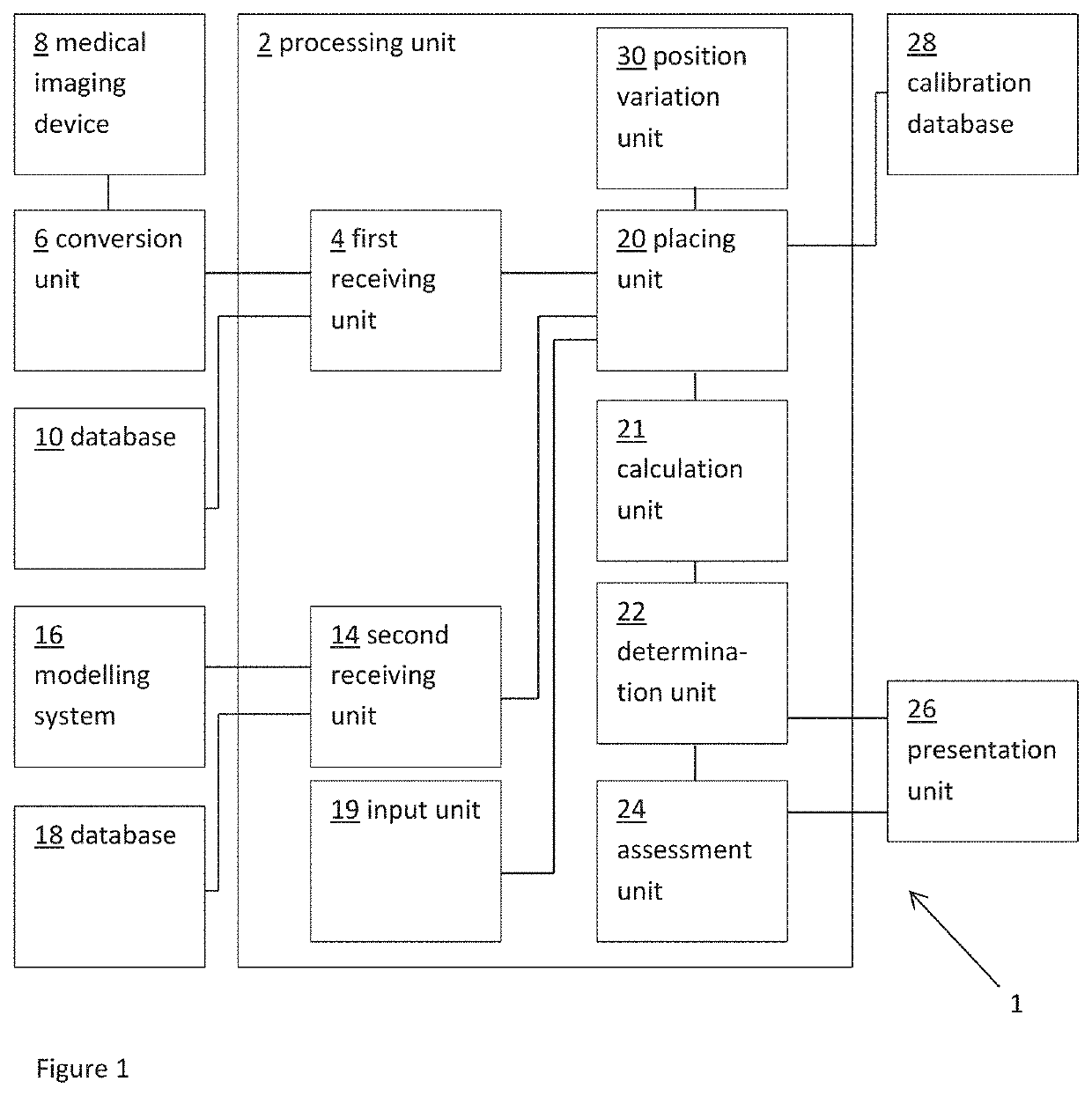
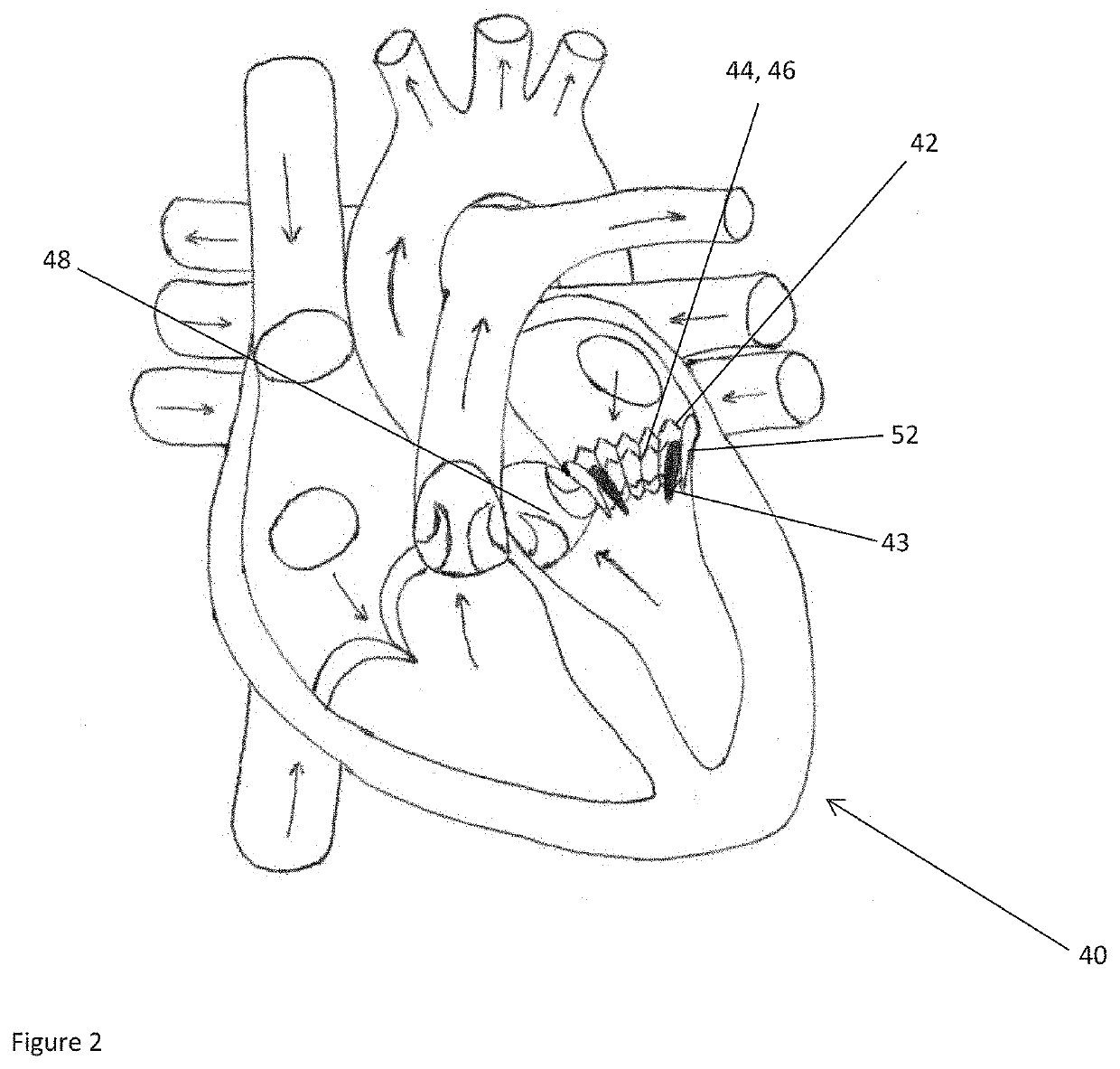
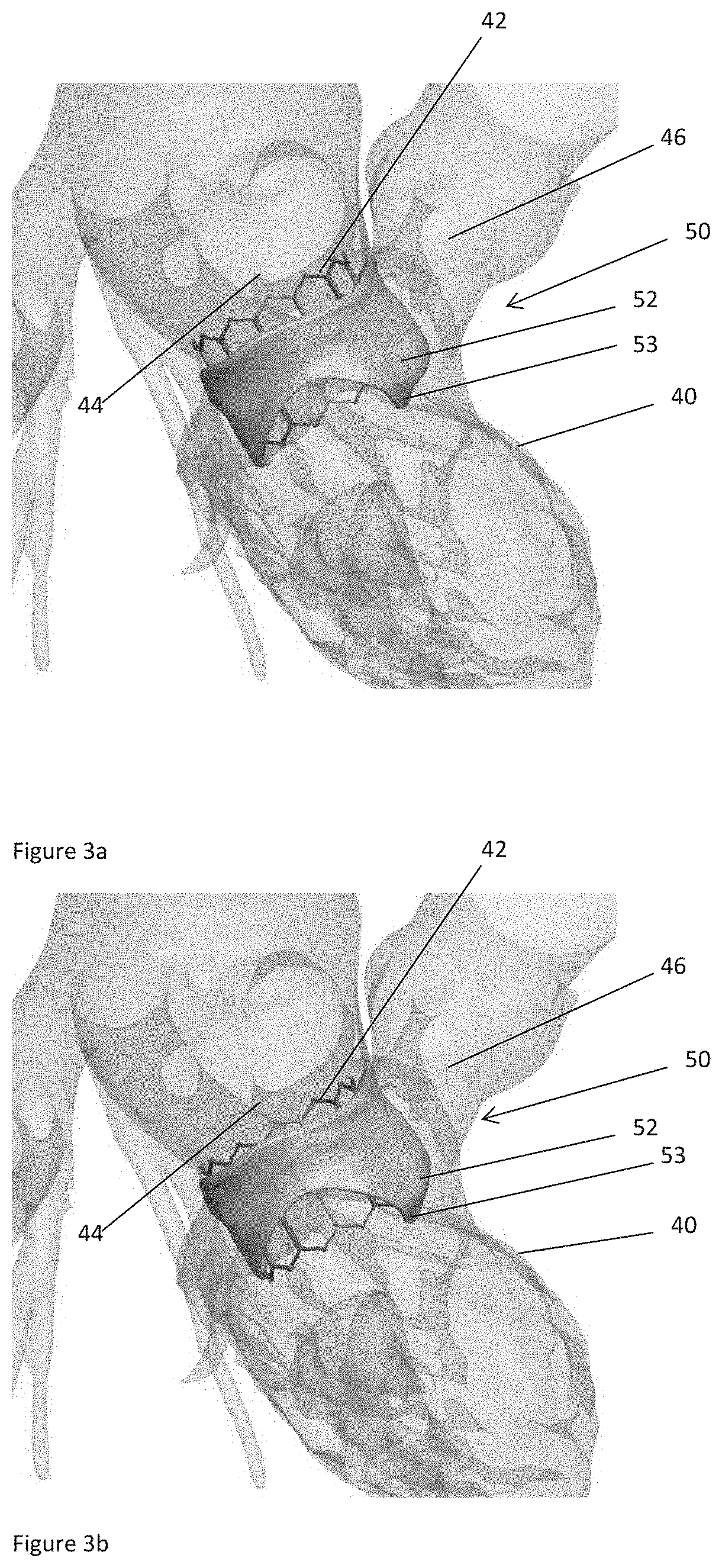
The present invention relates to a medical imaging system (10) for planning an implantation of a cardiac implant (42), comprising: a receiving unit (12) for receiving a plurality of three-dimensional (3D) cardiac images (14, 14′) showing different conditions of a heart (32) during a cardiac cycle; a segmentation unit (22) for segmenting within the plurality of 3D cardiac images (14, 14′) a target implant region (38) and a locally adjacent region (40) that could interfere with the cardiac implant (42); a simulation unit (24) for simulating the implantation of the cardiac implant (42) within the target implant region (40) in at least two of the plurality of 3D cardiac images (14, 14′); a collision evaluation unit (26) for evaluating an overlap (46) of the simulated cardiac implant (42) with the segmented locally adjacent region (40) in at least two of the plurality of 3D cardiac images (14, 14′); and a feedback unit (28) for providing feedback information to a user concerning the evaluated overlap (46).
Owner:KONINKLJIJKE PHILIPS NV
Intraoperative and post-operative adjustment of an annuloplasty ring
An intraoperative adjustment device is described. In some embodiments, the device includes an elongate body including a proximal end and a distal end, the distal end configured to penetrate an outer surface of an adjustable cardiac implant implanted in a patient's heart, and the proximal end and the distal end connected by at least one energy-transfer member. In some embodiments, the distal end includes at least one electrode coupled to the energy-transfer member and configured to deliver an activation energy to the adjustable cardiac implant. In some embodiments, the proximal end is configured to attach to an energy source that provides the activation energy. In some embodiments, the proximal end is configured to be located outside the patient's body while the distal end is coupled to the adjustable cardiac implant that is implanted in the patient's heart.
Owner:MICARDIA CORP
Planning an implantation of a cardiac implant
Owner:KONINKLJIJKE PHILIPS NV
Method and system for determining a risk of cardiac conduction abnormalities
ActiveUS20180289422A1High peak pressureHigh strainMedical simulationImage enhancementCatheterBiomedical engineering
A method and system for determining a measure of a risk of a patient developing cardiac conduction abnormalities as a result of transcatheter cardiac treatment. The method includes providing a patient-specific anatomical model representing cardiac region and an implant model representing a finite element representation of a cardiac implant. The method includes virtually placing said implant model into said patient-specific anatomical model. A measure of mechanical interaction between the implant model and the patient-specific anatomical model is determined and a measure of risk of the patient developing cardiac conduction abnormalities is determined on the basis of the determined mechanical interaction.
Owner:FEOPS NV
Method and system for determining a risk of hemodynamic compromise after cardiac intervention
ActiveUS11045256B2Avoid measuringReliable measurementMedical simulationMechanical/radiation/invasive therapiesBlood flowEngineering
A method and system for predicting a measure of hemodynamic compromise as a result of transcatheter cardiac treatment. The method includes providing a patient-specific anatomical model representing cardiac region and an implant model representing a three-dimensional representation of a cardiac implant. The method includes virtually deploying said implant model into said patient-specific anatomical model. A deformation of the patient-specific anatomical model is calculated as a result of implant model deployment A measure of hemodynamic compromise is determined from the virtually deployed implant model and the deformed patient-specific anatomical model.
Owner:FEOPS NV
Method and System for Determining a Risk of Hemodynamic Compromise After Cardiac Intervention
ActiveUS20190357981A1Reliable measurementAvoid measuringMedical simulationMechanical/radiation/invasive therapiesCatheterCvd risk
A method and system for predicting a measure of hemodynamic compromise as a result of transcatheter cardiac treatment. The method includes providing a patient-specific anatomical model representing cardiac region and an implant model representing a three-dimensional representation of a cardiac implant. The method includes virtually deploying said implant model into said patient-specific anatomical model. A deformation of the patient-specific anatomical model is calculated as a result of implant model deployment A measure of hemodynamic compromise is determined from the virtually deployed implant model and the deformed patient-specific anatomical model.
Owner:FEOPS NV
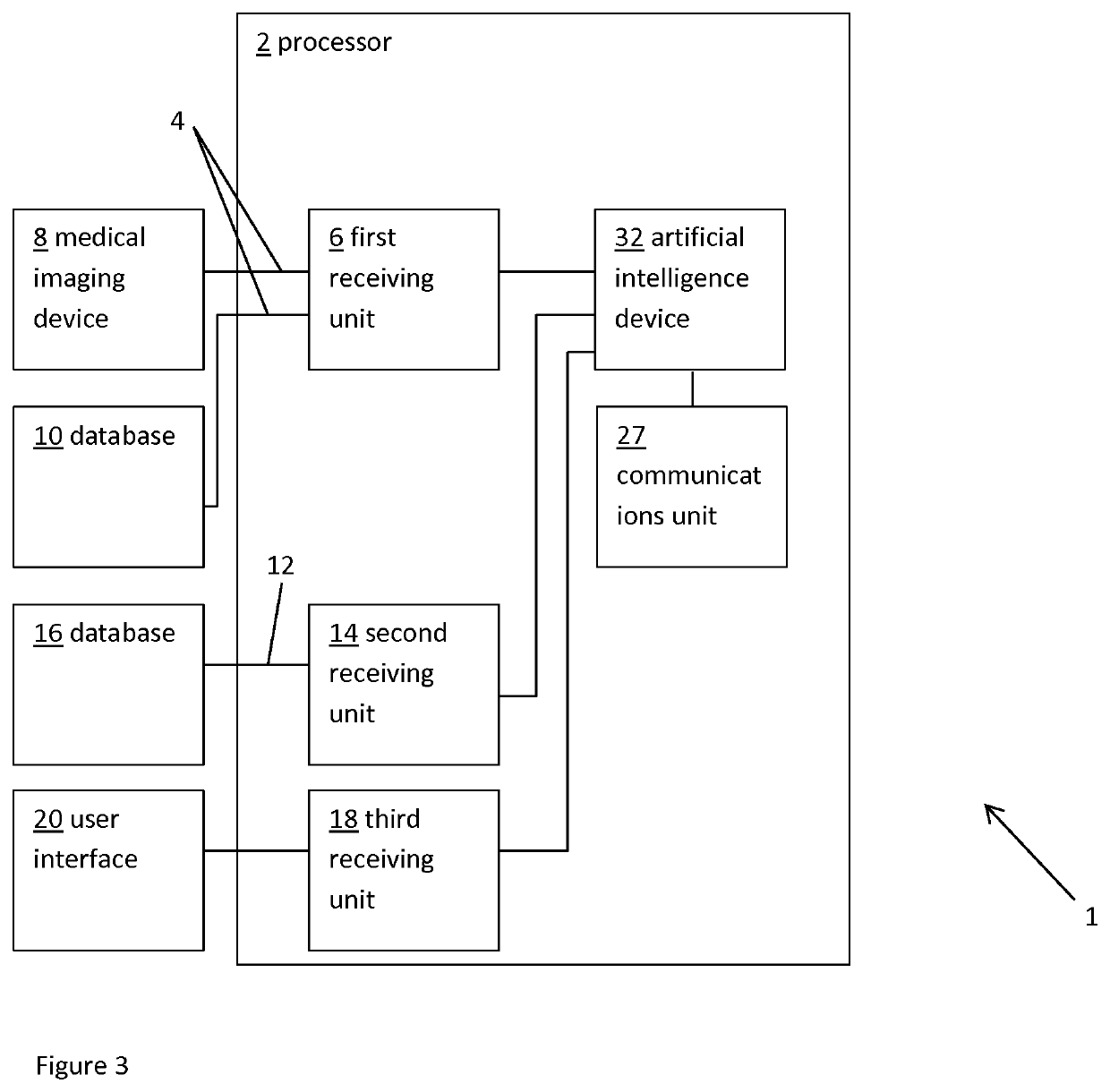
Method and system for patient-specific virtual percutaneous structural heart intervention
PendingUS20210022806A1Reliable measurementConvenient timeHeart valvesComputer-aided planning/modellingRat heartBiomedical engineering
A system and method for selecting, from a series of cardiac implants having different sizes, the cardiac implant having optimum size for implantation in a patient. The method includes obtaining data representative of a patient-specific cardiac region and predicting the optimum size of the cardiac implant best matching a predefined criterion when deployed in the cardiac region. The predicting includes querying a database; determining parameter values for a parametric model representation of the patient-specific cardiac region; and / or entering the data representative of the patient-specific cardiac region into an artificial intelligence device.
Owner:FEOPS NV
Cardiac implant delivery system
ActiveUS10426616B2Preferential bendingIncrease flexibilityStentsDiagnosticsDelivery systemBiomedical engineering
The present disclosure relates to delivery systems for delivering and deploying interventional devices to a targeted area within a body, such as delivering a replacement heart valve to a targeted heart valve. A delivery device includes a steerable catheter and a replacement valve delivery system positioned within the steerable catheter and configured to be translatable within the steerable catheter. The steerable catheter includes one or more control wires running from a distal end of the catheter to a handle at the proximal end of the catheter. Each control wire is coupled to a control of the handle such that manipulation of the control provides deflection and control of the steerable catheter.
Owner:EVALVE
Autonomous cardiac implant of the leadless capsule type, including a piezoelectric beam energy harvester
ActiveUS11357994B2Piezoelectric/electrostriction/magnetostriction machinesTransvascular endocardial electrodesMedicineEnergy harvester
An energy harvester includes a pendular unit subjected with a piezoelectric beam coupled to an inertial mass. On the clamped side of the beam, a beam frame includes two pressing elements between which the beam is taken in sandwich, each including i) an intermediate part, an internal face of which presses on a corresponding face of the beam, and ii) a pressure plate, an internal face of which presses on an external face of the intermediate part, a printed circuit board being interposed between them. The intermediate parts and the pressure plates are passed through by at least one common transverse bore receiving a locking pin. The intermediate parts, the pressure plates and the pin are each massive metal parts ensuring a direct electrical and mechanical contact with the electrodes of the beam and with the printed circuit boards.
Owner:CAIRDAC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com