Conjugates and their use as imaging agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
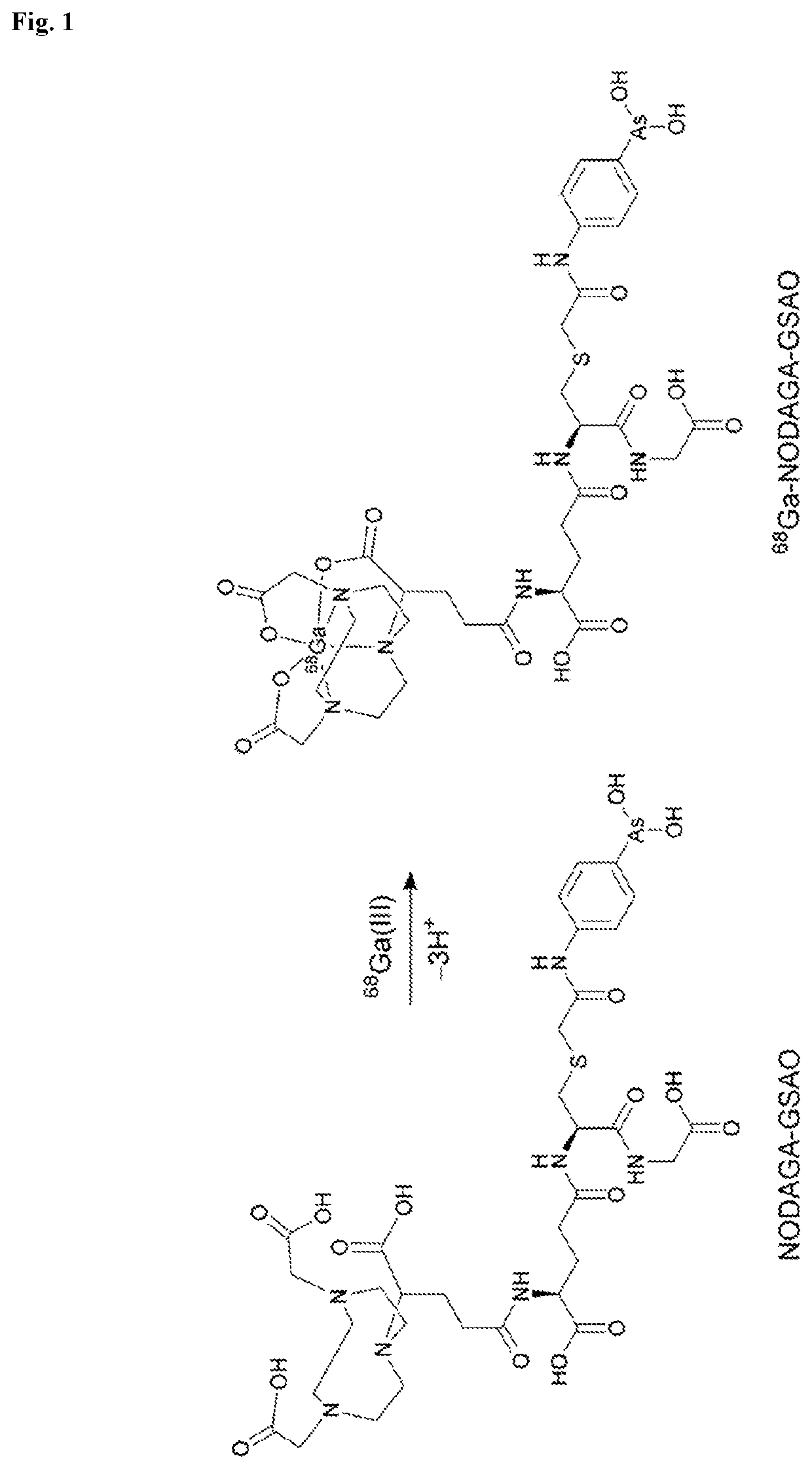
Synthesis of NODAGA-GSAO
[0111]a) GSAO was prepared using the process described in Park D, Don A S, Massamiri T et al (2011) Non-invasive imaging of cell death using an Hsp90 ligand. J Am Chem Soc 133:2932-3835; 4-(N-(bromoacetyl)amino)phenylarsonic acid (BRAA) was synthesized from p-arsanilic and bromoacetyl bromide, and BRAA reduced to 4-(N-(bromoacetyl)amino) phenylarsonous acid (BRAO). BRAO was coupled to glutathione (GSH) to produce GSAO. The GSAO was resolved from unreacted BRAO and GSH by C18 chromatography.
[0112]b) Sodium bicarbonate and ultrapure water were purged with nitrogen for 30 minutes prior to use. The reaction setup and purification were performed under an inert atmosphere of nitrogen. GSAO obtained from step a) (20.0 mg, 36.5 μmop was dissolved in 0.1 N sodium bicarbonate (7.4 mL) at 4° C. and stirred for 10 minutes.
[0113]c) NODAGA-NHS (2,2′-(7-(1-carboxy-4-((2,5-dioxopyrrolidin-1-yl)oxy)-4-oxobutyl)-1,4,7-triazonane-1,4-diyl)diacetic acid mono-N-hydroxysuccinimide...
example 2
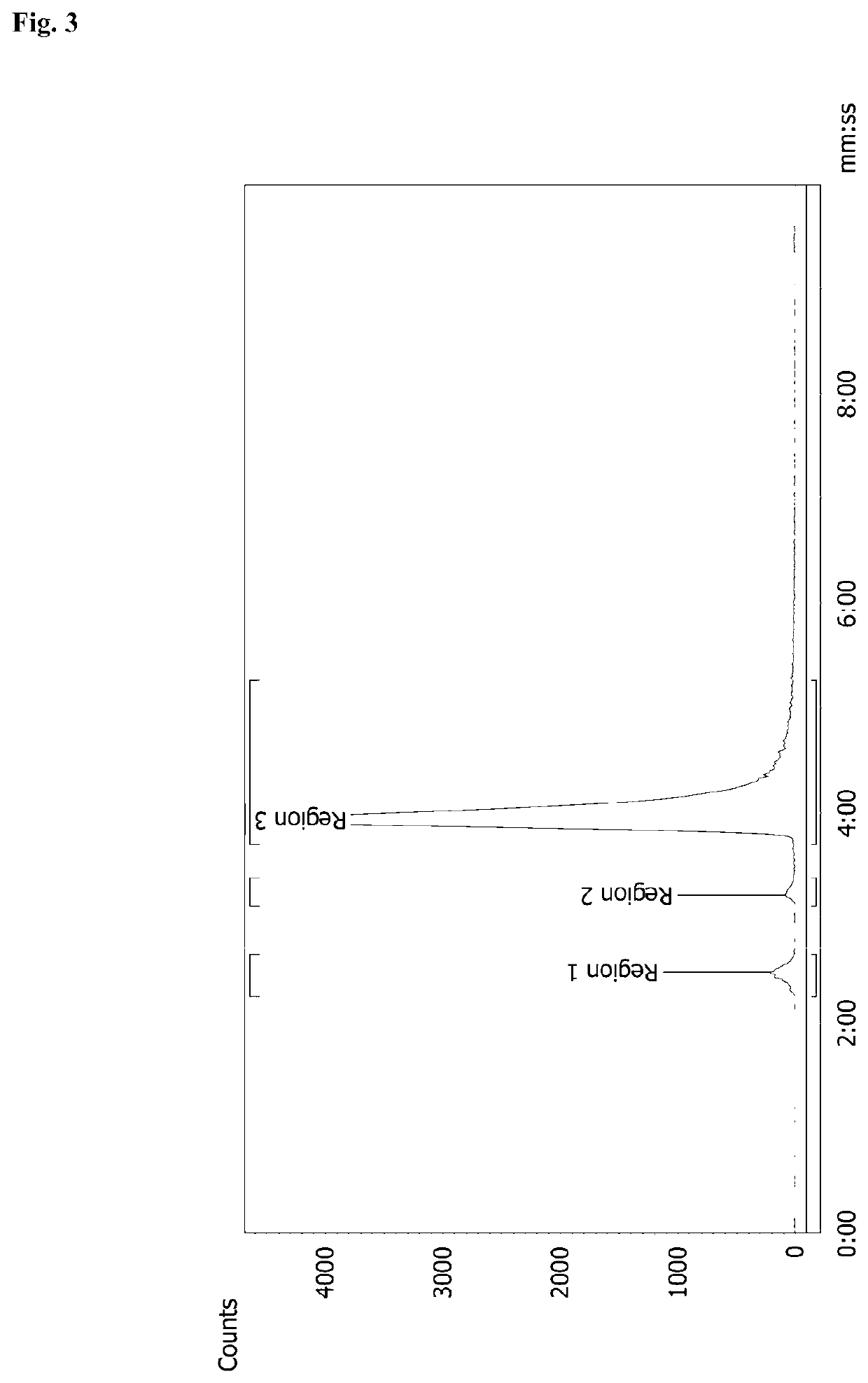
[0122]Radiolabelling of NODAGA-GSAO with 68Ga
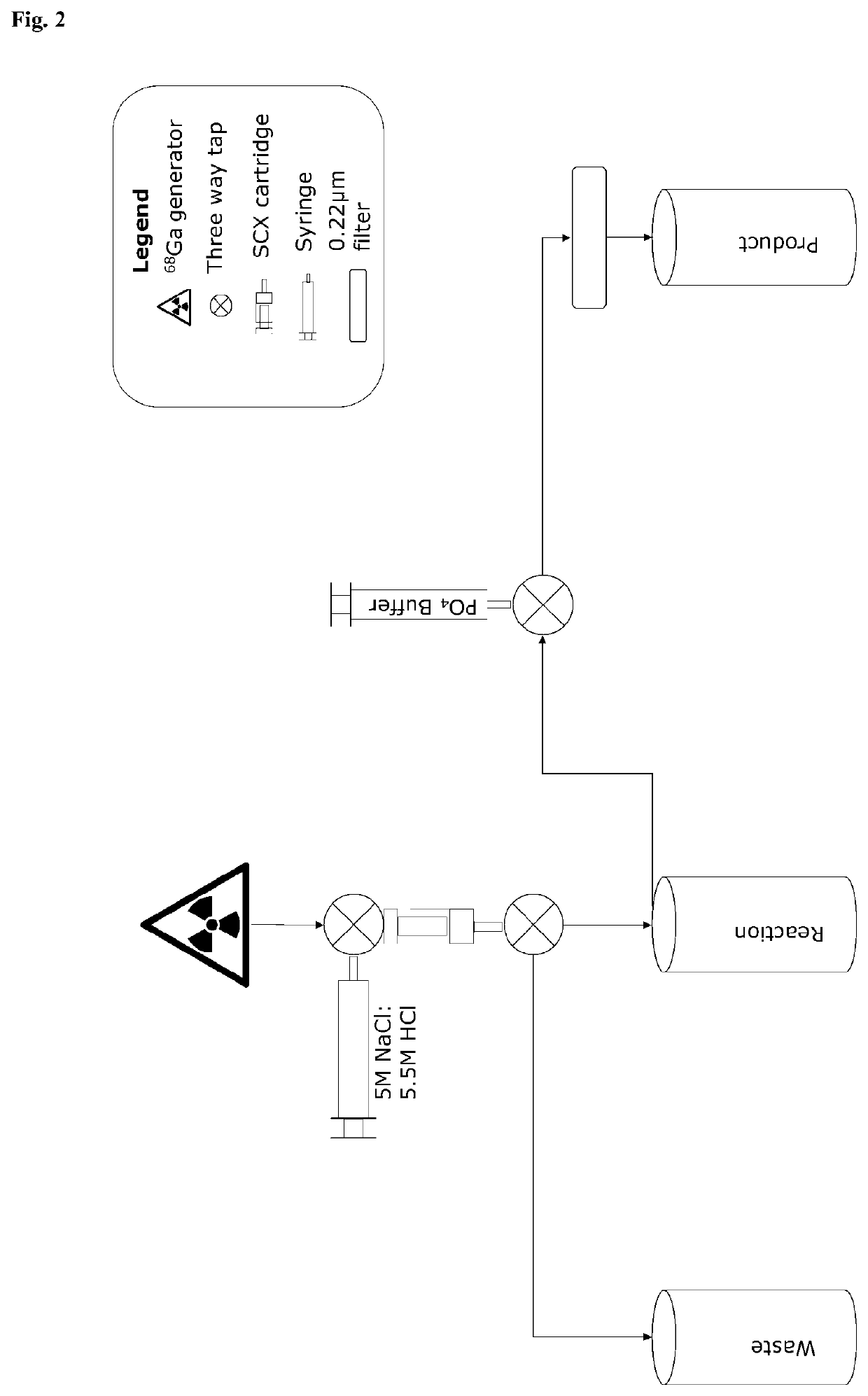
[0123]a) The barrel of the BondElute SCX column was cut so that when inserted the barbed female luer thread rested just above the column media to create a cartridge (hereafter referred to as the SCX cartridge). The barbed female luer thread should be fitted firmly and securely into the cut barrel of the BondElute SCX column to create a sealed cartridge that is air- and liquid-tight.
[0124]b) The SCX cartridge was primed with 1 mL 5.5 M HCl and then flushed with 10 mL of water.
[0125]c) The SCX cartridge was purged with air.
[0126]d) Ascorbic acid solution (0.25 M) was obtained by dissolving 44 mg of ascorbic acid in 1 mL water (Water Ultrapur, Merck).
[0127]e) A sodium acetate buffer (1.5 M CH3COONa.3H2O, pH4.5) was obtained by dissolving 10.21 g CH3COONa.3H2O in water (Water Ultrapur, Merck). The pH was adjusted to pH 4.5 with glacial acetic acid and water was added to a total volume of 50 mL
[0128]f) One vial of 54 μg NODAGA-GSAO obtained in...
example 3
Pharmaceutical Formulation of 68Ga-NODAGA-GSAO
[0139]A composition was prepared containing ingredients in the amounts listed in Table 1 below.
TABLE 1Ingredient NameQuantityUnitAscorbic acid4.4mgSodium95mgPhosphate109mgAcetate22mgChloride91mg68Ga-NODAGA-GSAO200MBq
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com