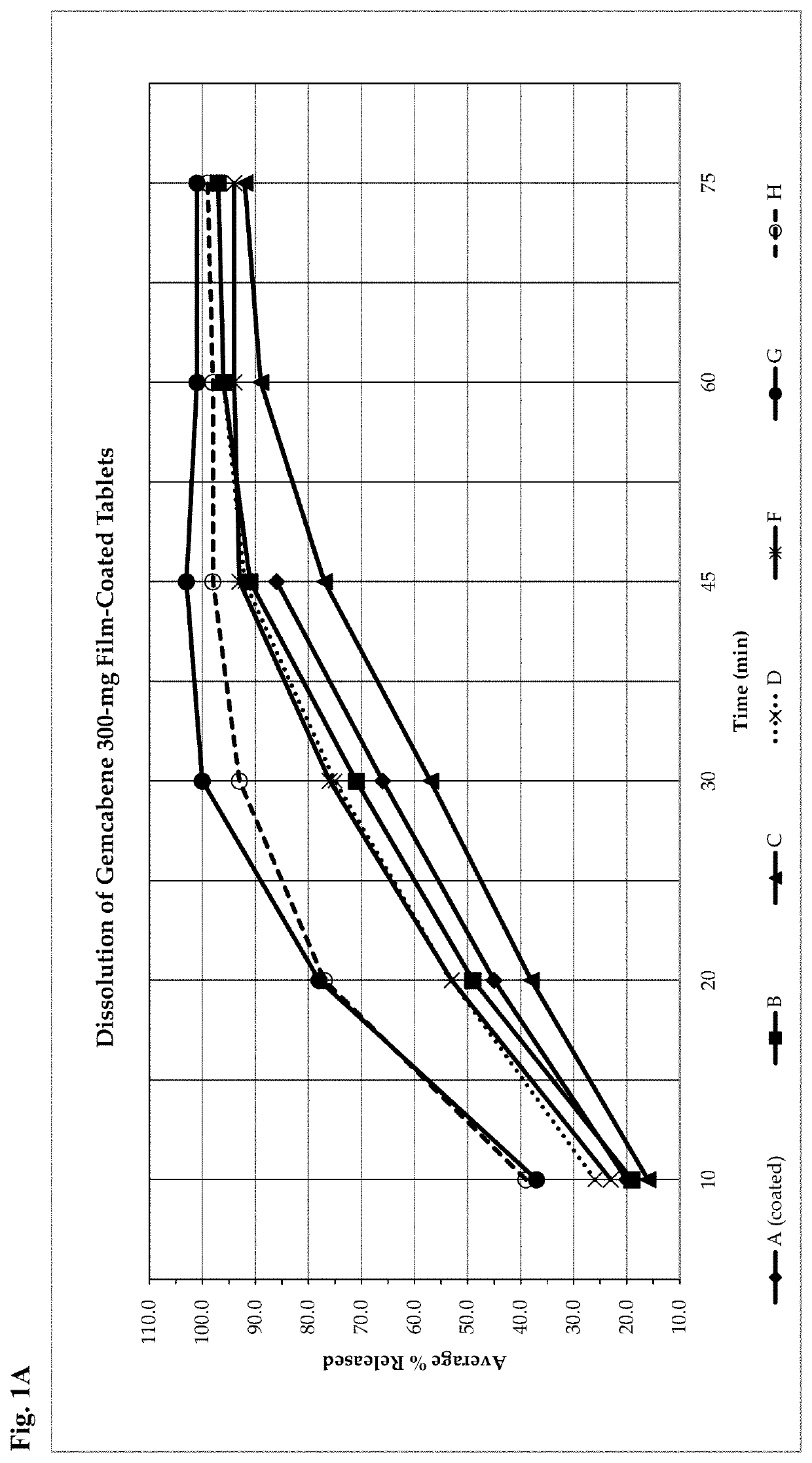
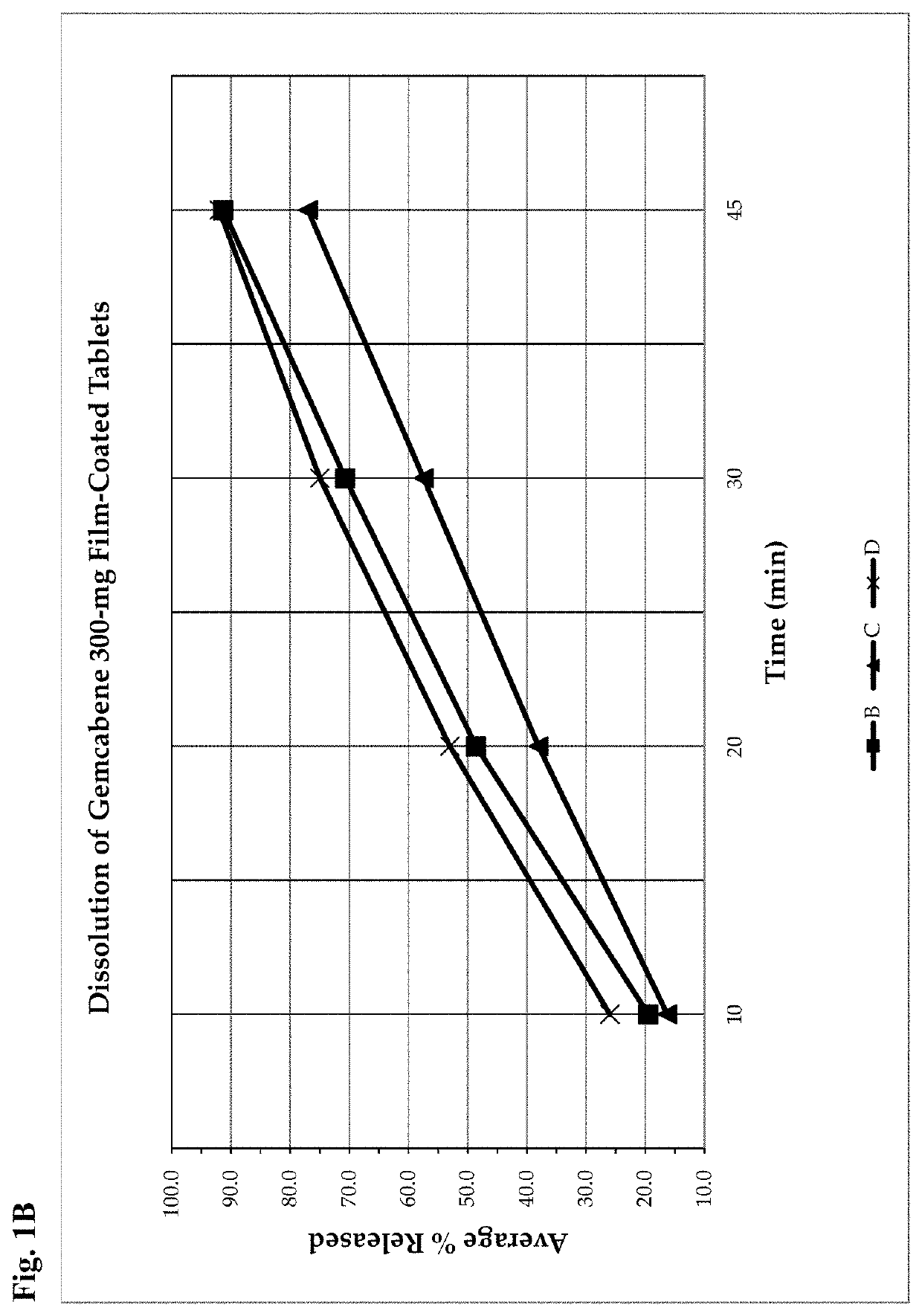
Gemcabene, pharmaceutically acceptable salts thereof, compositions thereof and methods of use therefor
a technology of methylhexanoic acid and gemcabene, which is applied in the field of pharmaceutically acceptable salts of 6(5carboxy5methylhexyloxy)2, 2dimethylhexanoic acid, can solve the problems of high risk of non-alcoholic fatty liver disease (nafld) and non-alcoholic steatosis, liver damage, and swelling of the liver, so as to prevent or reduce the risk of pancreatitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Gemcabene Calcium Salt Hydrate Crystal Form 1
[0593]
Step 1. 6-(5-Carboxy-5-Methyl-Hexyloxy)-2,2-Dimethylhexanoic Acid (Gemcabene)
[0594]In a reactor (ST-1005, glass-lined, 1600 l), isobutyric acid (41.0 kg, 466 mol, 2.2 equiv) and heptane (276 kg) were combined and a molar equivalent of 30% sodium hydroxide was charged (62.1 kg), followed by water (1.1 kg) and heptane (126 kg) under stirring. The mixture was refluxed with water removal until the rate of water removal effectively stopped. Then, a Karl-Fisher analysis of the water content was performed to confirm removal of water (water content measured 0.012%). Tetrahydrofuran (THF) (279 kg) was added followed by a lithium diisopropylamide solution (lithium diisopropylamide 28% w / w in heptane / THF / ethylbenzene, 174.6 kg, 2.2 equiv) at 10° C.-15° C. After flushing with THF (33.8 kg) the mixture was heated at 42° C.±2° C. for about 1 hour. Bis-(4-chlorobutyl)ether (42.0 kg, 211 mol, 1.0 equiv, BCBE) diluted with THF (11.6 kg)...
example 2
y Studies of Gemcabene Calcium Salt Crystal Form 1
[0682]Approximately 20 mg of gemcabene calcium Crystal Form 1 was added to 5×2 mL vials. The solubility in 5 solvents was tested using a solvent addition method. Solvents included acetone, ethanol, ethyl acetate, t-butyl methyl ether (t-BME) and water. Solvent was added in 5 volume (100 μL) aliquots until either dissolution or 2 mL in total had been added. Between each addition, samples were heated to 60° C. (40° C. for acetone and t-BME). Any solids remaining after 24 hours at ambient were analyzed by X-ray powder diffraction (XRPD). Water sample dissolved and did not precipitate even after 48 hours at <5° C. Table 1 shows the result of the solubility studies.
TABLE 1Solubility of gemcabene calcium salt Crystal Form 1SolventSolubility (mg / mL)Crystalline FormAcetoneForm 1EthanolForm 1Ethyl AcetateForm 1 / -Butyl Methyl Ether (t-BME)Form 1Water33N / A
example 3
Gemcabene Calcium Salt
[0683]Gemcabene calcium salt Crystal Form 1 was prepared as described in Example 1. Approximately 40 g of gemcabene calcium salt Crystal Form 1 was weighed. To this, approximately 800 mL of water was added and mixed at ambient temperature for dissolution. After approximately 4 hours, the solid was found to have dissolved and the solution was transferred to a 2 L round bottom flask. The solution was then frozen before being placed on a freeze dryer for approximately 72 hours. X-ray powder diffraction (XRPD) analysis of a combined lot of material showed that the diffractogram is consistent with reference amorphous data (FIG. 52A). Polarized light microscope (PLM) images showed glass-like particles with limited birefringence. Thermogravimetric analysis (TGA) showed a weight loss of 3.1% up to 150° C. (FIG. 52B). No thermal events were noted in the differential thermal analysis (DTA) or in the differential scanning calorimetry (DSC) (FIGS. 52B and 52C). The moistur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com