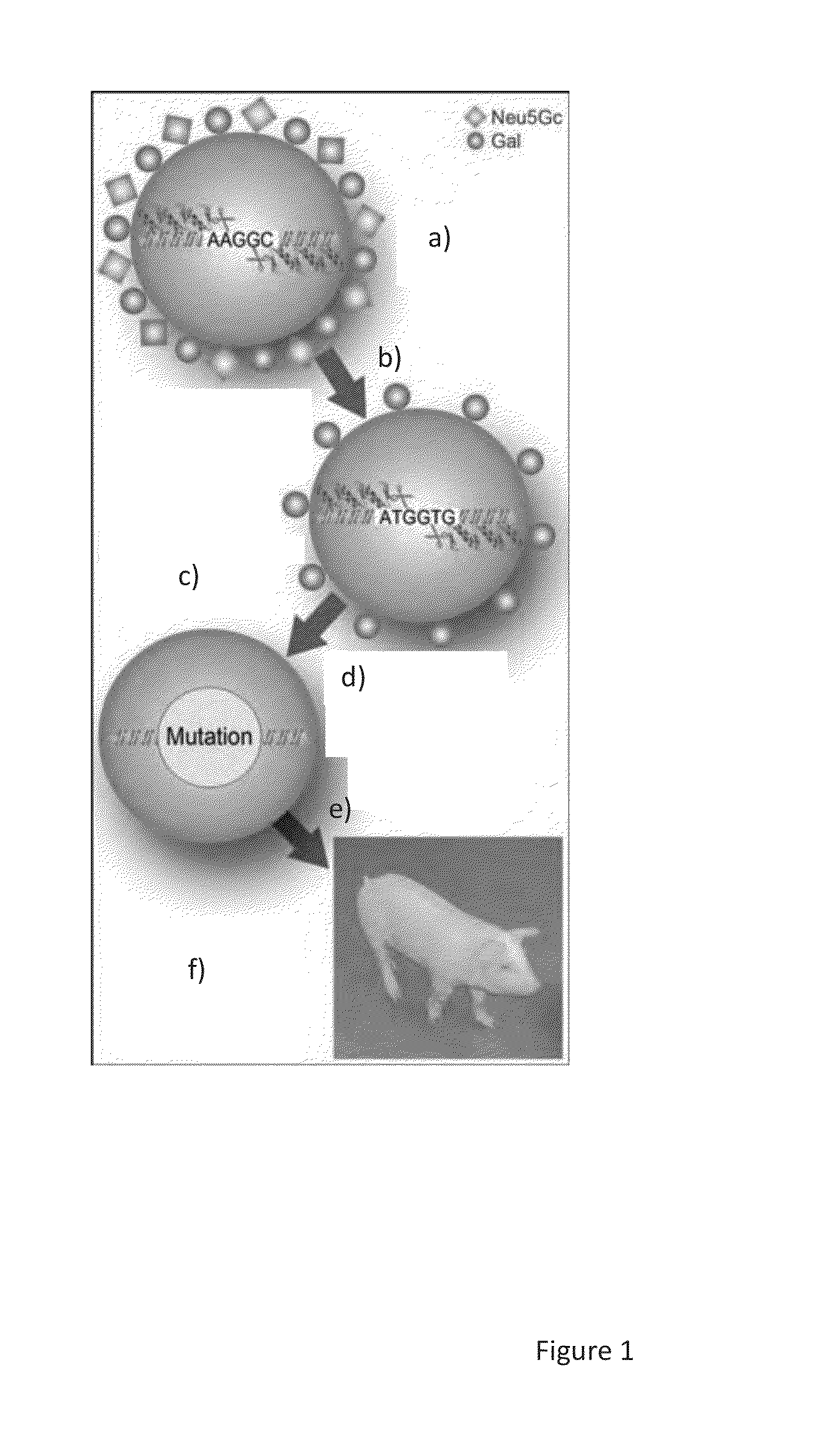
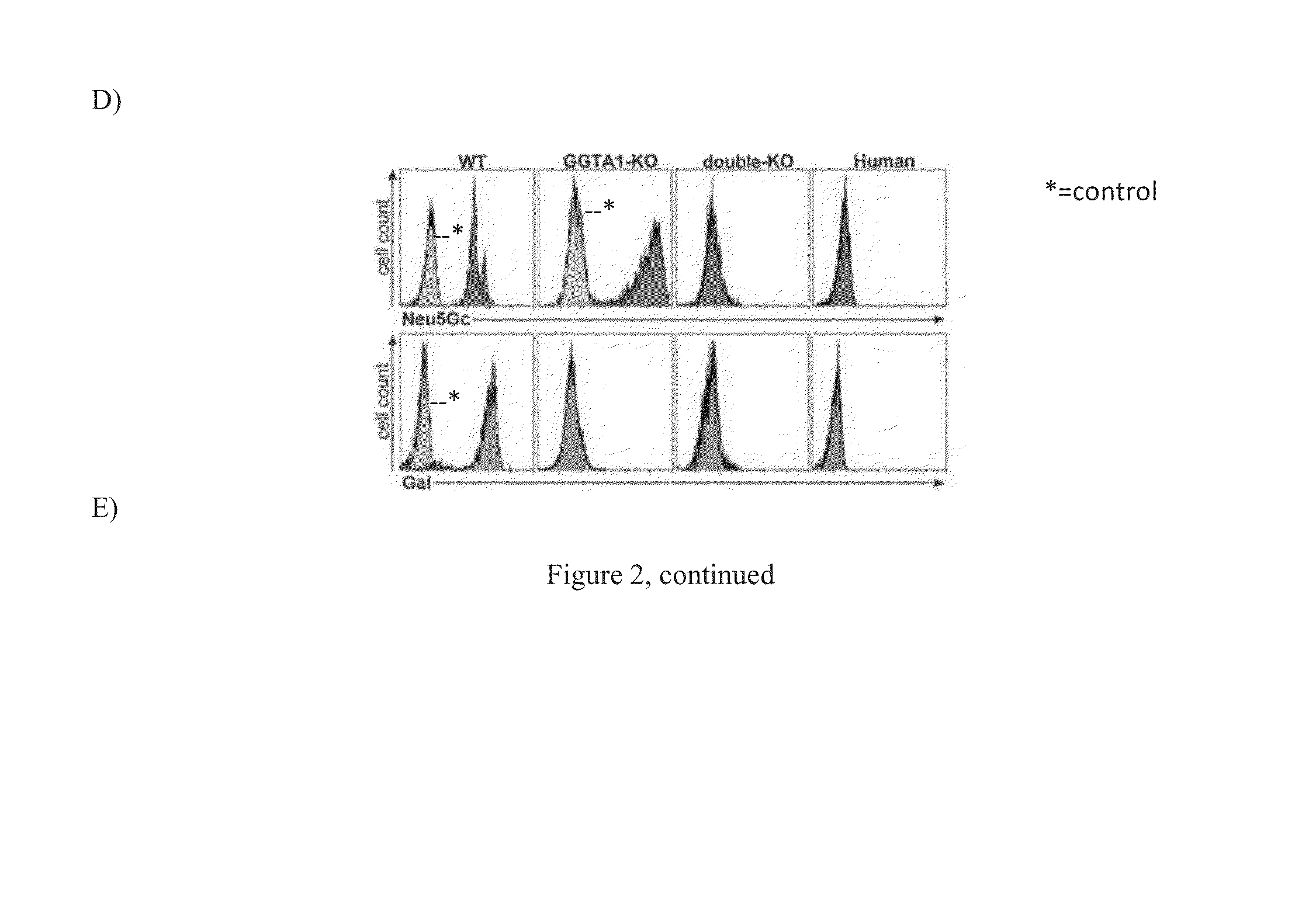
Double knockout (gt/cmah-ko) pigs, organs and tissues
a technology of pigs and organs, applied in the field of xenotransplantation and genetic modification, can solve the problems of severe rejection of transplanted tissue, unmodified pig tissue into human (or other primate), and insufficient suitable organs for transplantation, so as to improve the symptoms of hyperacute rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Zinc Finger Nucleases (ZFN)
[0059]A pair of ZFN were designed to bind and cleave the sequence of porcine CMAH exon 9 (SEQ ID NO: 1-AAACTCCTGAACTACAAGGCTCGGCTGGTGAAGGA) beginning at position 1,341 of Ensemble transcript ENSSSCT00000001195. Another pair of ZFN were designed to bind and cleave the region of GGTA1 exon 8 (SEQ ID NO: 2-GTCATCTTTTACATCATGGTGGATGATATCTCCAGGATGCC) beginning at position 1165 of Ensemble transcript ENSSSCT00000006069. The ZFN activities were validated in yeast (Sigma-Aldrich, St. Louis, Mo.). Additional detail regarding the ZFN of the present invention can be found in Li et al., Journal of Surgical Research, (2012)E1-E7 Epub 2012 Jul. 3, which is incorporated by reference herein for all purposes.
example 2
Cell Culture and Transfection of Porcine Liver Derived Cells
[0060]Porcine adult liver derived cells (LDC) were isolated with modifications from the method described in Li et al, 2010 Cell Reprogram. 12:599. Isolated LDC were cultured in a combination stem cell media (SCM) (α-MEM:EGM-MV 3:1) (Invitrogen / Lonza, Switzerland) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, Utah), 10% horse serum (Invitrogen, Carlsbad, Calif.), 12 mM HEPES, and 1% Pen / Strep (Invitrogen) as described in Li et al 2012 JSR Epub 2012 Jul. 3. A commercial porcine strain (Landrace / York / Chester White) was used as the source of the LDC. Neon transfection system was used according to the manufacturer's instruction (Invitrogen).
[0061]Briefly, LDC were harvested by trypsin digestion, washed with calcium and magnesium free Dulbecco's phosphate buffered saline (DPBS) (Invitrogen) and centrifuged. 106 cells were suspended in 120 μl of resuspension buffer (Invitrogen) containing 2 μgs of each CMAH ZFN p...
example 3
Surveyor Mutation Detection Assay (CEL I Assay)
[0063]ZFN-induced mutation was detected using the Surveyor Mutation Detection kit (Transgenomic, Omaha, Nebr.). At day 5 post-transfection, genomic DNA from ZFN-treated and control plasmid pEGFP-N1 treated cells was extracted and PCR was performed using primers ZFN-CMAH-F (SEQ ID NO: 3-5′ GGACCTGCTTTATCTTGCTCGC 3′), ZFN-CMAH-R (SEQ ID NO: 4-5′ CCATACTTGTCTGCTGGGTGGG 3′). Pwo SuperYield DNA Polymerase, dNTPack (Roche, Indianapolis, Ind.) was used and the PCR conditions were as follows: 94° C., 2 minutes; 94° C., 15 seconds, 55° C., 30 seconds and 68° C., 50 seconds for 15 cycles; 94° C., 15 seconds, 5 5° C., 30 seconds and 68° C., 50 seconds with additional 5 seconds for each cycle, for 25 cycles and a final extension step of 68° C. for 5 minutes.
[0064]PCR product was denatured and annealed using the following program on a MyCycler (Bio-Rad): 95° C., 10 minutes; 95° C. to 85° C., −2° C. / second; 85° C. to 25° C., −0.1° C. / second. 200-400 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| mean fluorescence intensities | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com