Surfaces containing coupling activator compounds and reinforced composites produced therefrom
a technology of activator compounds and surface, which is applied in the direction of weaving, transportation and packaging, ceramic layered products, etc., can solve the problems of high glass loading of glass-reinforced polyamide composites, and achieve the effects of improving interfacial adhesion, tougher composite materials, and improving the coupling between glass and polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
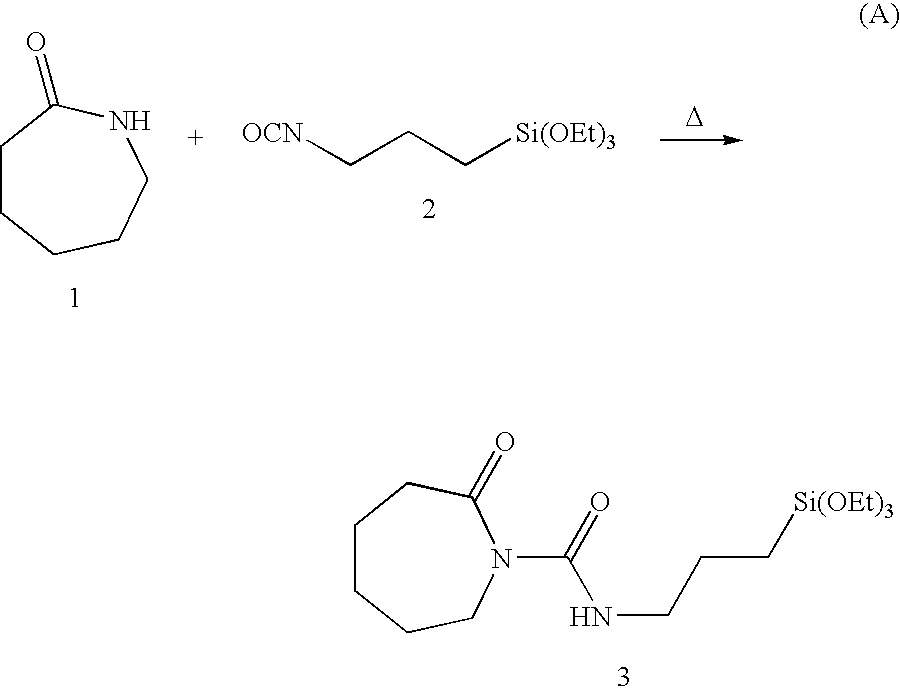
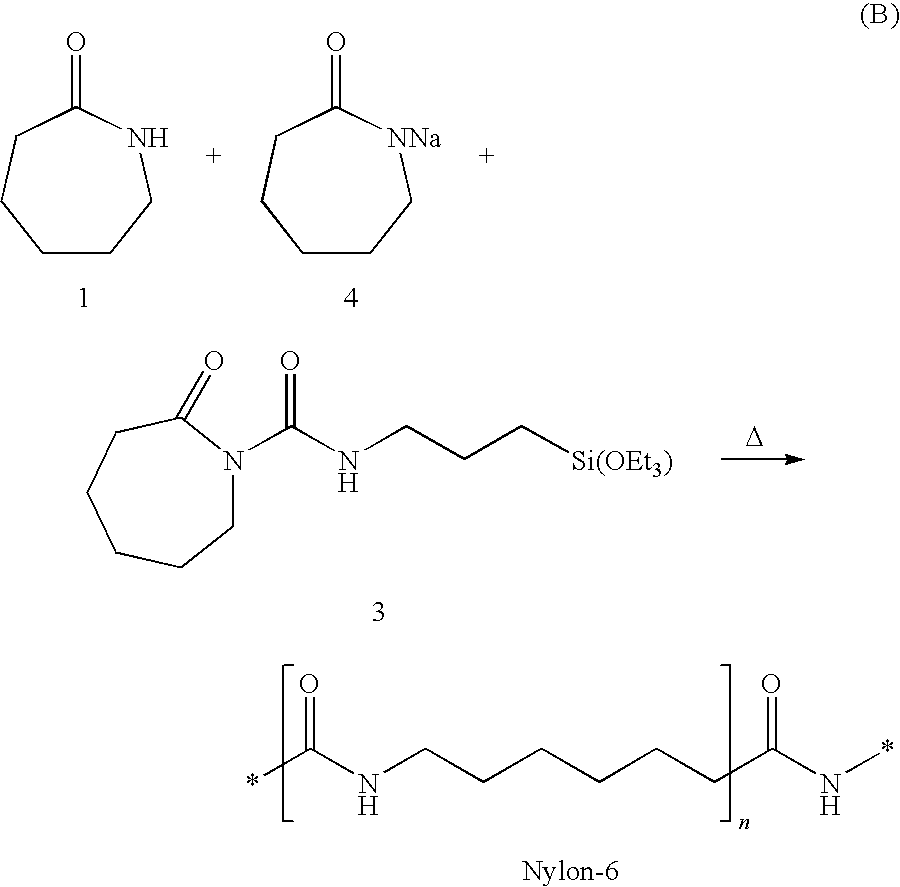
[0037]Chopped fibre strands sized with a sizing composition comprising 2-oxo-N-(3-(triethoxysilyl)propyl)azepane-1-carboxamide (compound 3 in reaction scheme A above) may be fed into an extruder as previously described. A monomer mix comprising caprolactam monomer 1 and sodium caprolactam catalyst 4, as shown in reaction scheme B above, may also be fed into the extruder to be mixed and heated with the sized glass fibres. The processing conditions within the extruder initiate and complete an anionic ring-opening polymerization of caprolactam 1 in accordance with reaction scheme B, and strands of the resulting glass-reinforced Nylon-6 may be extruded through the extruder die. A sample strand of the glass-reinforced Nylon-6 may be broken under tension. The breaking point may be analyzed with a Scanning Electron Microscope (SEM) to show the outstanding coupling of glass and polymer in the composite material provided by the present invention.
example 2
[0038]Chopped glass fibres strands may be sized with a conventional sizing composition comprising 0-30 wt % of γ-aminopropyltriethoxysilane or other suitable silane coupling agent, 20-70 wt % of a polyurethane emulsion or a suitable mixture of emulsions, and 10-50 wt % of a lubricant or mixture of lubricants, and 0-50 wt % of any other required or preferred additives. The chopped sized fibres may be fed into the same extruder used in Example 1 above. Referring to reaction scheme E below, monomer mix comprising caprolactam monomer 1, sodium caprolactam catalyst 4 and a commercially-available activator 5 may also be fed into the extruder, thereby mixing and heating the mix with the sized glass fibres. The processing conditions within the extruder initiate and complete an anionic ring-opening polymerization of the caprolactam monomer 1 within the extruder in accordance with reaction scheme E below:
[0039]Strands of the resulting Nylon-6 may then be obtained from the extruder die and ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Shape | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com