Ga-base alloy and organic function element using the same
a technology of organic function elements and alloys, applied in the field of metal materials for electrode formation, can solve the problems of high oxidizability and high combustibility, increase in vacuum devices and deposition masks, and difficulty in handling alkali metals and alkaline earth metals, etc., to achieve the effect of reducing production costs, low production costs, and increasing the size of organic function elements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(1) Preparation of Coating Liquid for Organic EL Layer Formation
[0153] Coating liquids having the following compositions for organic EL layer formation was prepared. In the compositions of the coating liquids, a fluorescent colorant was varied to prepare three coating liquids having different luminescent colors. When the fluorescent colorant is Coumarin 6, green luminescence having a peak at 501 nm is obtained; when the fluorescent colorant is perylene, blue luminescence having a peak at 460 to 470 nm; and when the fluorescent colorant is DCM (dicyanomethylene pyran derivative), red luminescence having a peak at 570 nm is obtained. These were used as luminescent materials for respective colors.
[0154]
Polyvinyl carbazole70parts by massOxadiazole compound30parts by massFluorescent colorant1part by mass(Coumarin 6, perylene or DCM)Monochlorobenzene (solvent)4900parts by mass
(2) Preparation of Metal for Electrode Formation
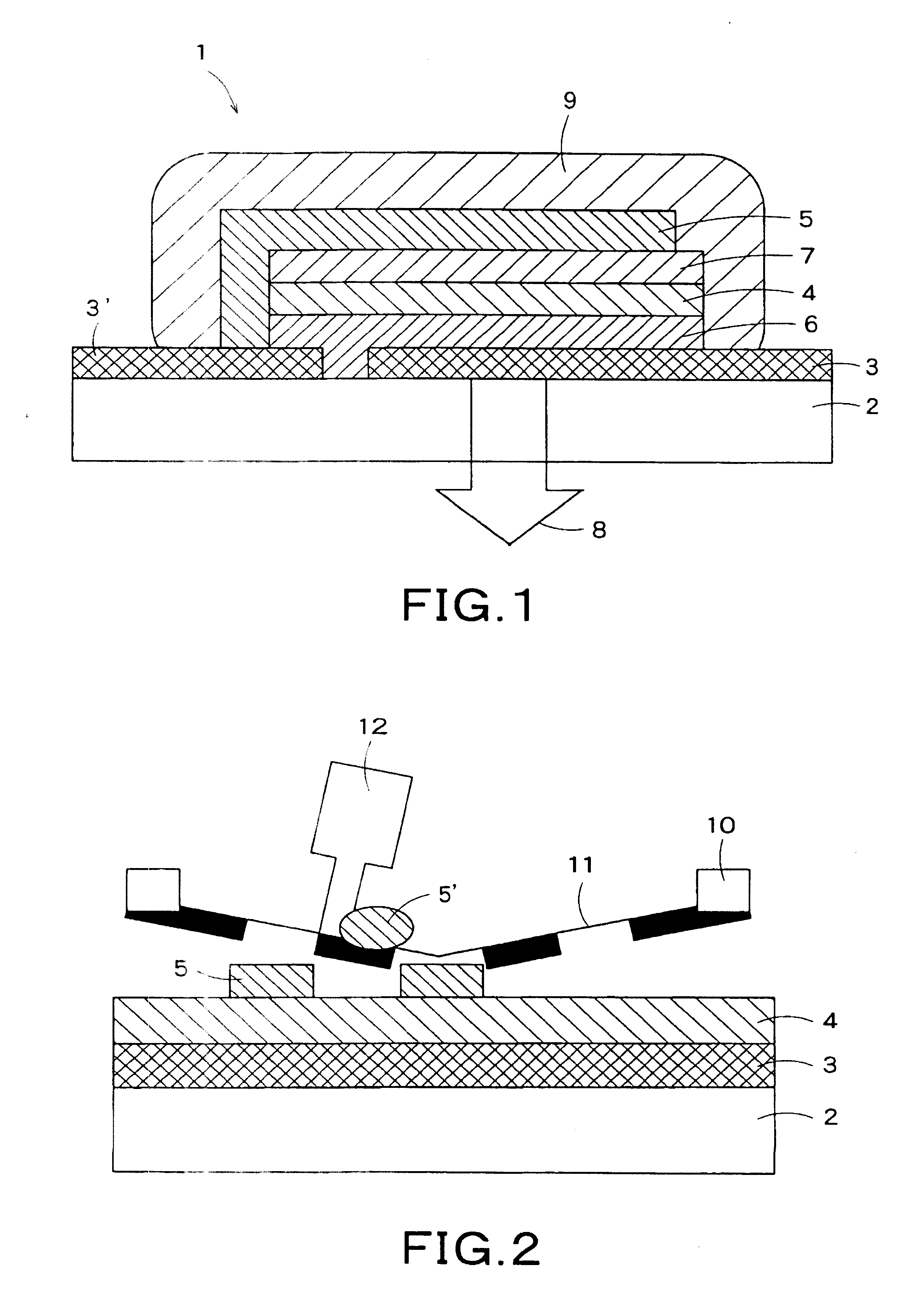
[0155] An alloy of Ca (calcium) and In (indium) at a molar ra...
example 2
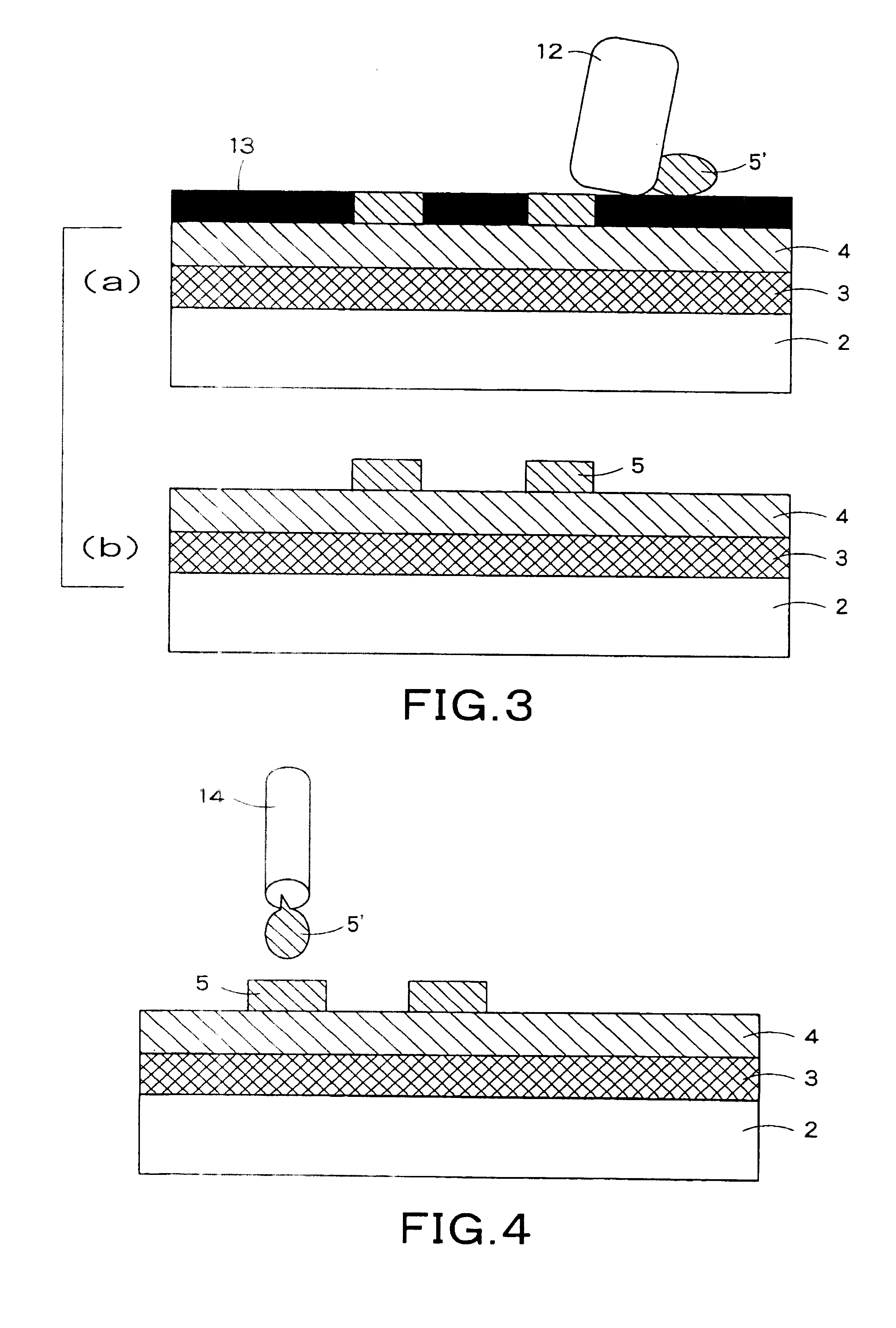
[0169] An element was prepared in the same manner as in Example 1, except that the concentration of Ca in the Ga-base alloy used in Example 1 was changed to 30% by mass. The viscosity of the Ga-base alloy was measured with a rotational viscometer and was found to be 100 Pa·s. However, the viscosity of the Ga-base alloy used in this Example was higher than that of the Ga-base alloy used in Example 1, and, in the screen printing, the Ga-base alloy paste disadvantageously clogged the mesh holes, making it impossible to form a cathode. For this reason, the same electrode pattern as in Example 1 was printed using a stainless steel metal mask. As a result, the cathode could be formed. Subsequently, in the same manner as in Example 1, the electrode of the Ga-base alloy was covered with the adhesive for fixation and sealing to prepare an organic EL element.
[0170] The organic EL element thus prepared was DC-driven using ITO as an anode and the electrode of the Ga-base alloy as a cathode. As...
example 3
[0172] Elements were prepared in the same manner as in Examples 1 and 2, except that the Ga-base alloy used in Examples 1 and 2 was coated by a dispenser method to form a cathode. In the dispenser method, a metal paste having a wide concentration range can be coated by regulating the nozzle bore diameter, ejection air pressure, and temperature to form a cathode.
[0173] The organic EL elements thus prepared were DC-driven using ITO as an anode and the electrode of the Ga-base alloy as a cathode. As a result, for both the organic EL elements, luminescence started at 2.0 to 2.2 V. At 4.2 to 4.4 V, the luminescence intensity was 100 cd / m2 in terms of luminance, and at 5.8 to 6.0 V, the luminescence intensity was 1000 cd / m2 in terms of luminance. The time necessary for the luminance to be reduced by 50% when the initial luminance was 1000 cd / m2, that is, half-life period, was measured and was found to be 100 hr.
[0174] The above results show that, for the organic EL elements prepared in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com