Method for preparing refractory active metal or alloy
An active metal and refractory technology, applied in the field of metallurgy of rare earth metals, heavy rare earth metals and their alloys, can solve the problems of electrochemically polarized small metals, achieve short production processes, reduce energy consumption, and avoid secondary The effect of secondary electrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
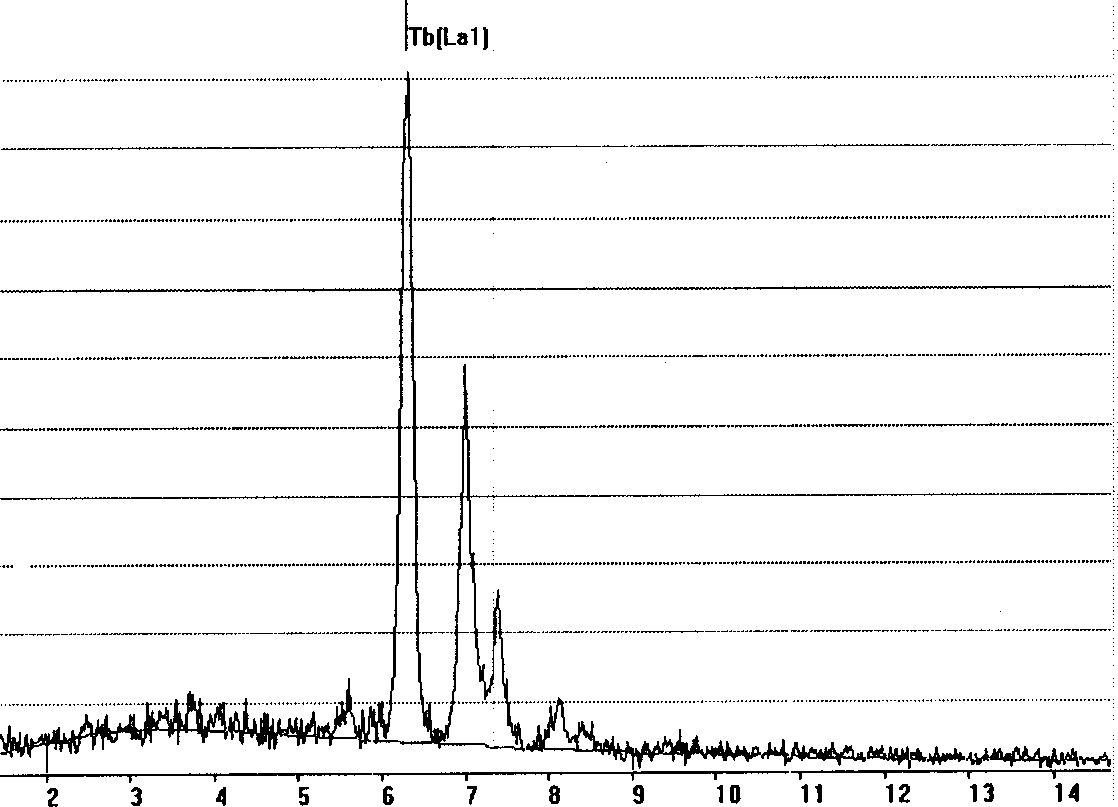
[0044] Tb with a purity of 99.99% 4 o 7 The powder is mixed with water, pressed into blocks at 2-5MPa, sintered at 1000°C in air for 1-4 hours, and the porosity of the sample is 30-48% ( figure 1 ). Weigh 5-10g sintered body, put it in molybdenum / tungsten / tantalum basket, drop to CaCl 2 The melt is used as the cathode, and the graphite crucible is used as the anode. In an argon atmosphere, at 900°C, the cell pressure is controlled at 3.4V, and the electrolysis is performed for 5-18 hours. The electrolysis product can be smelted by vacuum to remove the salt attached to the product. The impurity CaCl in the product can also be washed out by dimethyl sulfoxide 2 . After its electrolysis product was washed with dimethyl sulfoxide solvent, SEM ( figure 2 ) to observe its morphology and EDAX analysis components ( image 3 ), confirmed that the spongy rare earth metal terbium was obtained by electrolysis.
Embodiment 2
[0046] Dy with a purity of 99.9% 2 o 3 The powder is mixed with water, pressed into blocks at 2MPa, and sintered in air at 700°C for 5 hours, and the porosity of the sample is 50-60%. Weigh 5-10g sintered body, put it in molybdenum / tungsten / tantalum basket as cathode in CaCl 2 - Using graphite rod as anode in NaCl mixed melt, in argon atmosphere, electrolyzing at 650°C with a cell pressure of 4.5V for 5-18 hours. The electrolysis product was confirmed to be Dy metal element ( Figure 4 ), the oxygen content is 1000-5000ppm.
Embodiment 3
[0048] Tb with a purity of 99.99% 4 o 7 powder with 10wt% NH 4 HCO 3 Mix well, press into blocks at 2-5MPa, pre-fire in air at 300°C for 1-3 hours, then heat up to 1000°C and sinter for 2-4 hours to obtain a sample with a porosity of 65-75%. Filled with CaCl at 600°C 2 + LiCl mixed molten salt titanium crucible, bound Tb with molybdenum wire 4 o 7 The powder sintered block is used as the cathode, and the graphite is used as the anode, and the electrolysis is carried out at a controlled voltage of 4.2V for 8-16 hours in an argon atmosphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com