Process for preparing recombined placental growth factor
A technology of placental growth factor and form, which is applied in the field of preparation of recombinant placental growth factor, and can solve the problems of not describing conditions and experimental details
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: fermentation
[0052] The following steps relate to the method of fermentation and induction of a genetically modified microorganism (MOGM) [Bl21(DE3)pLysS PLGF-1] in a fermentation vessel using 1 mM IPTG.
[0053] Material:
[0054] Solution SBM consists of the following:
[0055] Solution A (per 1 liter)
[0056] Bacto Yeast Extract (Difco) 34g
[0058] Glycerin 100ml
[0059] h 2 O about 900ml
[0060] Solution B (10X) (per 100ml)
[0061] K H 2 PO 4 1.7g
[0062] K 2 HPO 4 -3H 2 O 20g, or
[0063] K 2 HPO 4 15.26g
[0064] h 2 O add appropriate amount to 100ml
[0065] Solutions A and B were autoclaved separately and mixed under aseptic conditions for use. Alternatively, solutions A and B are mixed under aseptic conditions and filtered.
[0066] IPTG 200mM (200X) was prepared by dissolving 5g of pure material in 100ml of distilled water. The solution was filtered through a 0.22 μm filter, subdivi...
Embodiment 2
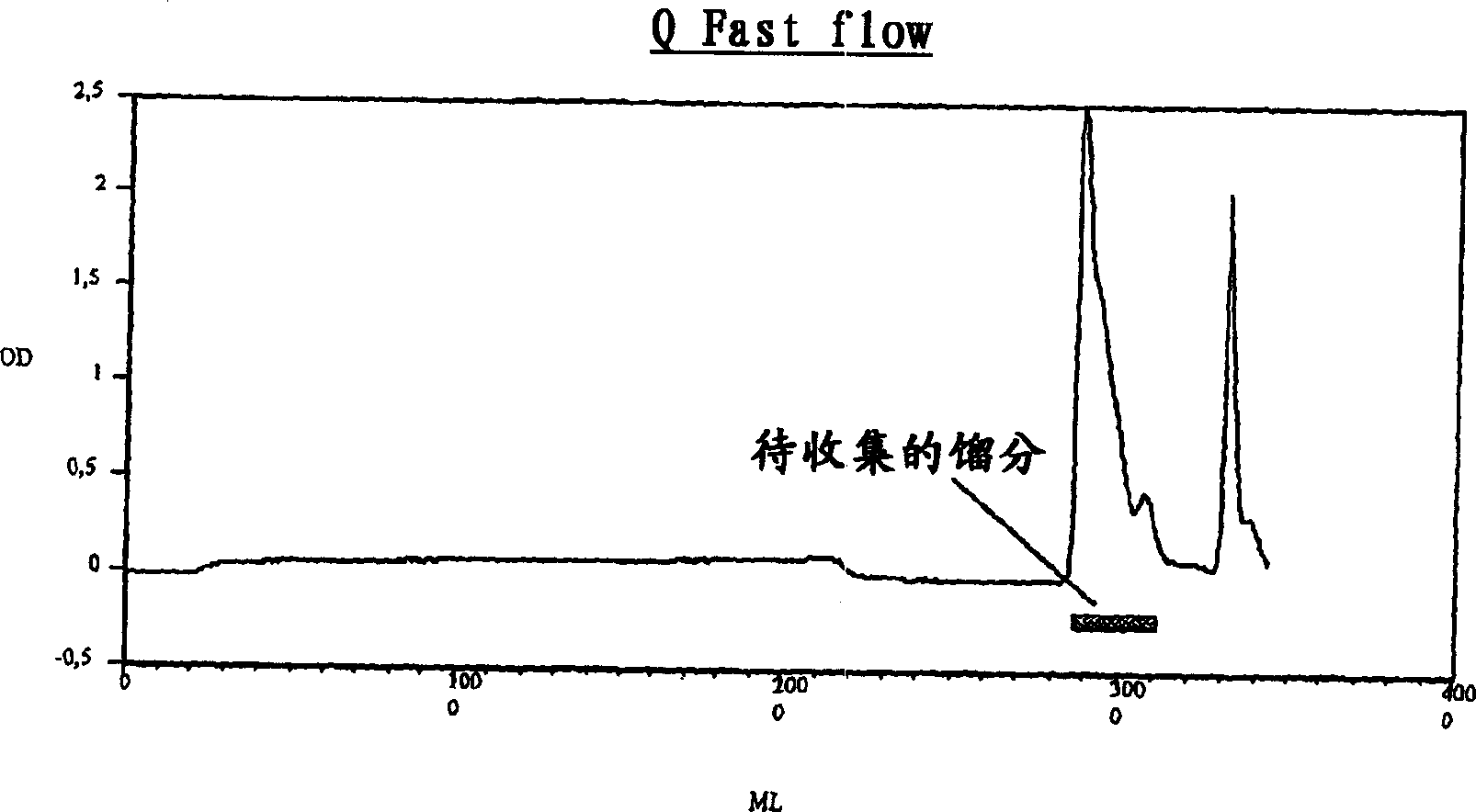
[0096] Example 2: Extraction and purification of inclusion bodies
[0097] The following steps involve the preparation and refolding of inclusion bodies of PLGF-1. Upon refolding, the PLGF-1 bacterial protein is partially restored to the dimeric form.
[0098] Material:
[0099] Mixer of appropriate capacity
[0100] Lysis solution: 1mM Mg 2 SO 4 +20mM Tris-HCl pH8+1%TritonX100.
[0101] Washing solution: 0.5% triton X100+10mM EDTA pH8.
[0102] BD (denaturing buffer): 8M urea, 50mM Tris pH8, ethylenediamine 20mM.
[0103] dissolve in water.
[0104] Oxidized Glutathione 200x: 100mM, in water
[0105] Reduced Glutathione 200x: 250mM in water.
[0106] Dilution buffer: PEG 4000 (2.4g / l) at a final concentration of 600uM
[0107] 50mM Tris-HCl pH8, 20mM NaCl.
[0108] Antifoaming agent: Antifoam O-10 (non-silicon) Sigma
[0109] Preparation of PLGF-1 inclusion bodies.
[0110] Lysis and wash solutions were equilibrated at room temperature (RT).
[0111] Freeze / thaw...
Embodiment 3
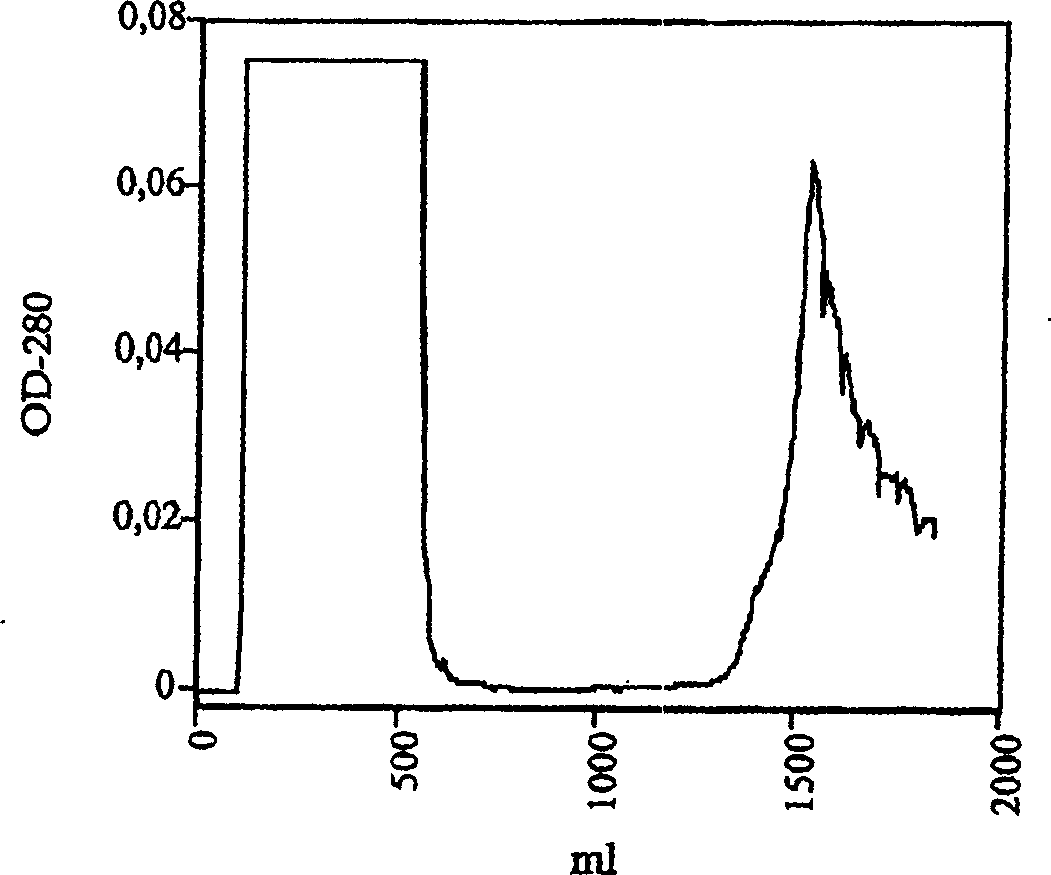
[0122] Example 3: Renaturation of protein
[0123] Inclusion bodies were dissolved in 7ml of denaturing buffer BD (containing urea 8M) and further diluted in BD until OD280 was 0.8. Subsequently, 0.6 volumes of dilution buffer were added in order to bring the final concentration of urea to 5M.
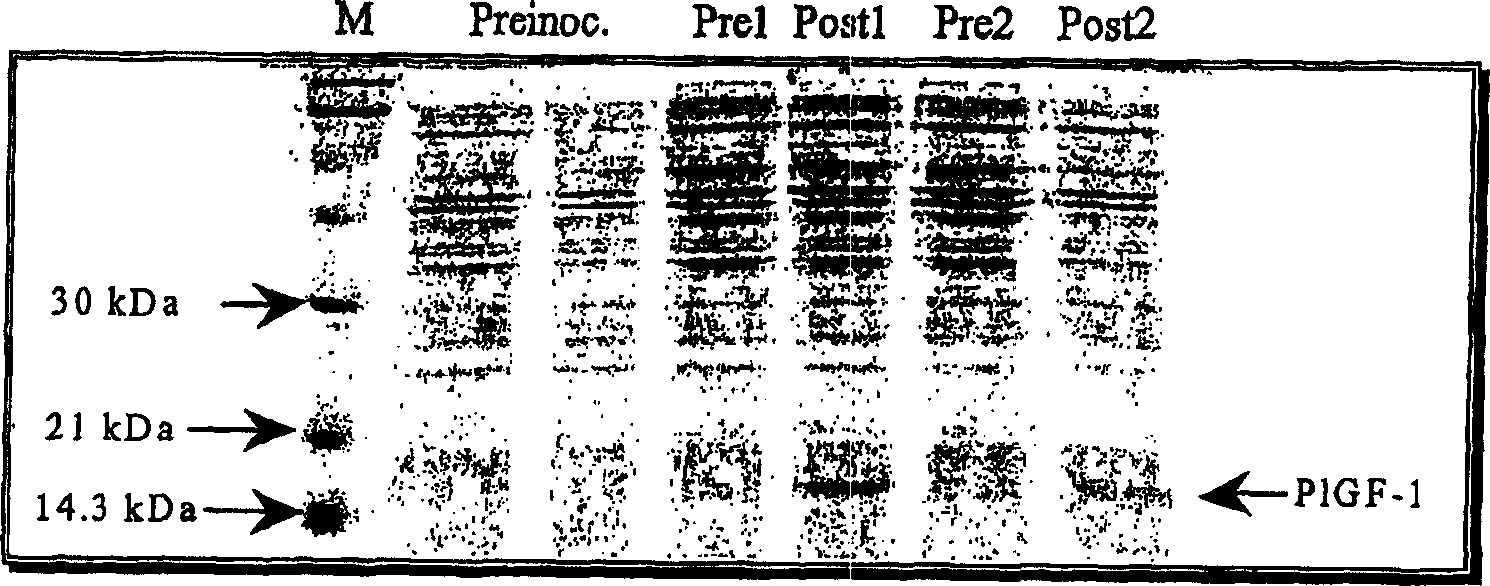
[0124] Thereafter, 1 / 200 reduced glutathione 200× (final concentration 1.25 mM) and 1 / 200 oxidized glutathione 200× (final concentration 0.5 mM) were added. 15 μl of the sample for examination (prerefol) was taken, and the solution was then incubated at 20° C. with stirring for 18-20 hours.
[0125] At the end of the incubation period, the medium was centrifuged at 10.000 x g for 10 minutes at 20° C., filtered against a 0.45 or 0.8 μm filter and a 15 μl sample was taken for inspection (postrefol).
[0126] Results: 15 μl of the solution before and after refolding were analyzed by SDS-PAGE electrophoresis ( Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com