Method for comprehensively utilizing phosphate fertilizer by-product
A by-product and phosphate fertilizer technology, applied in the field of phosphate fertilizer by-product utilization, can solve the problems that product quality cannot meet market requirements, restrict the development of phosphate fertilizer industry, and cannot be effectively used, and achieve low equipment material requirements, easy industrial production, well-designed effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
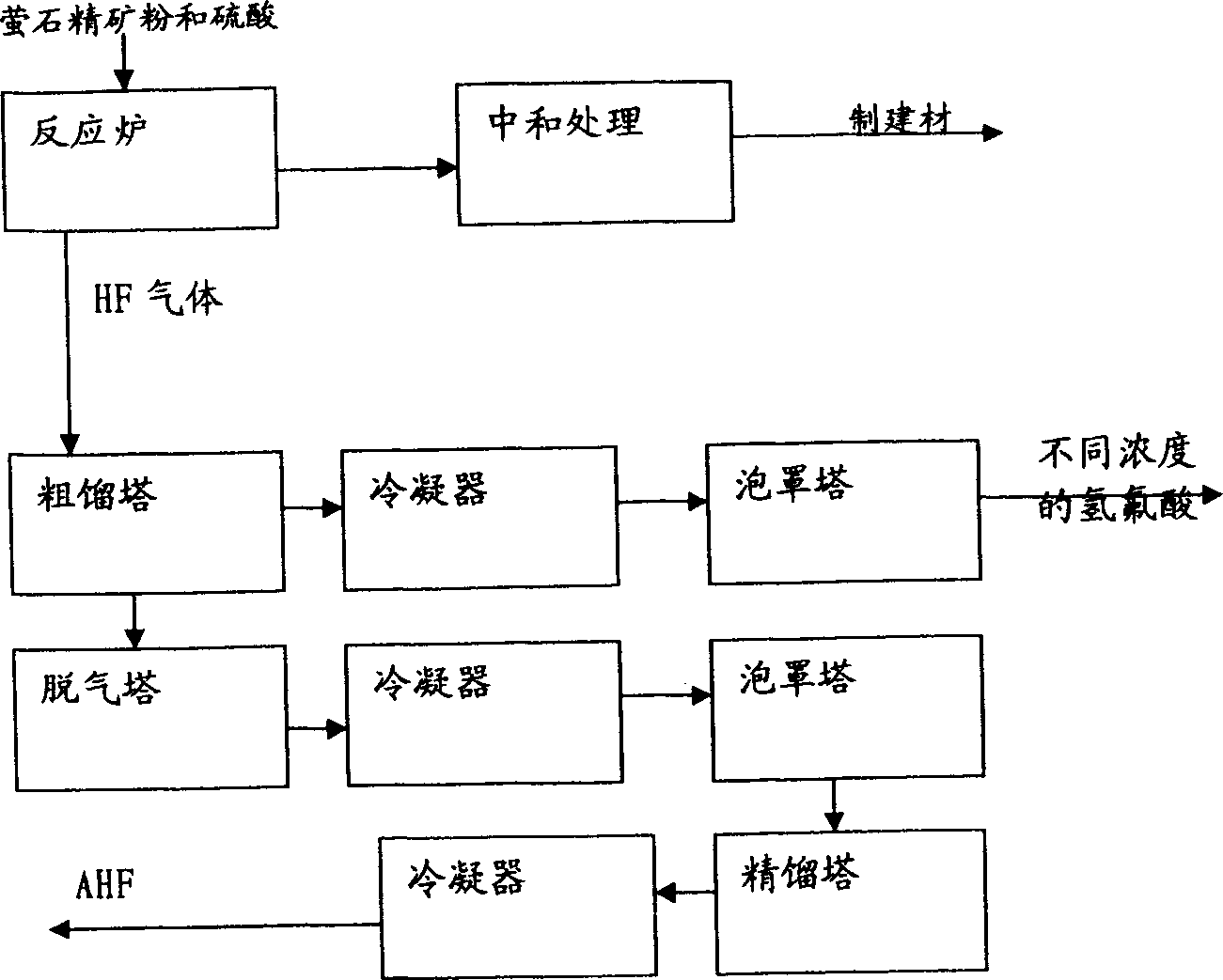
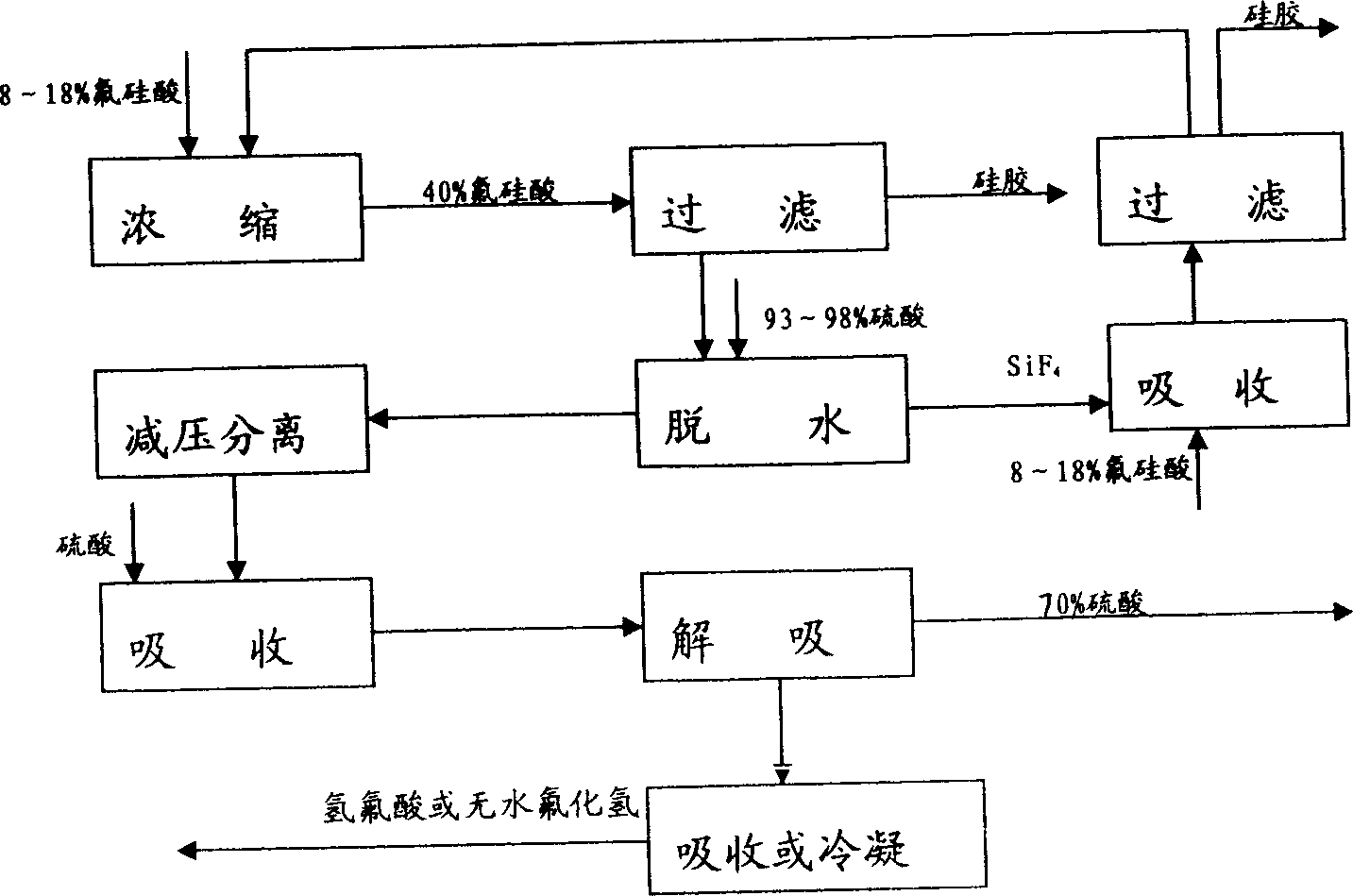
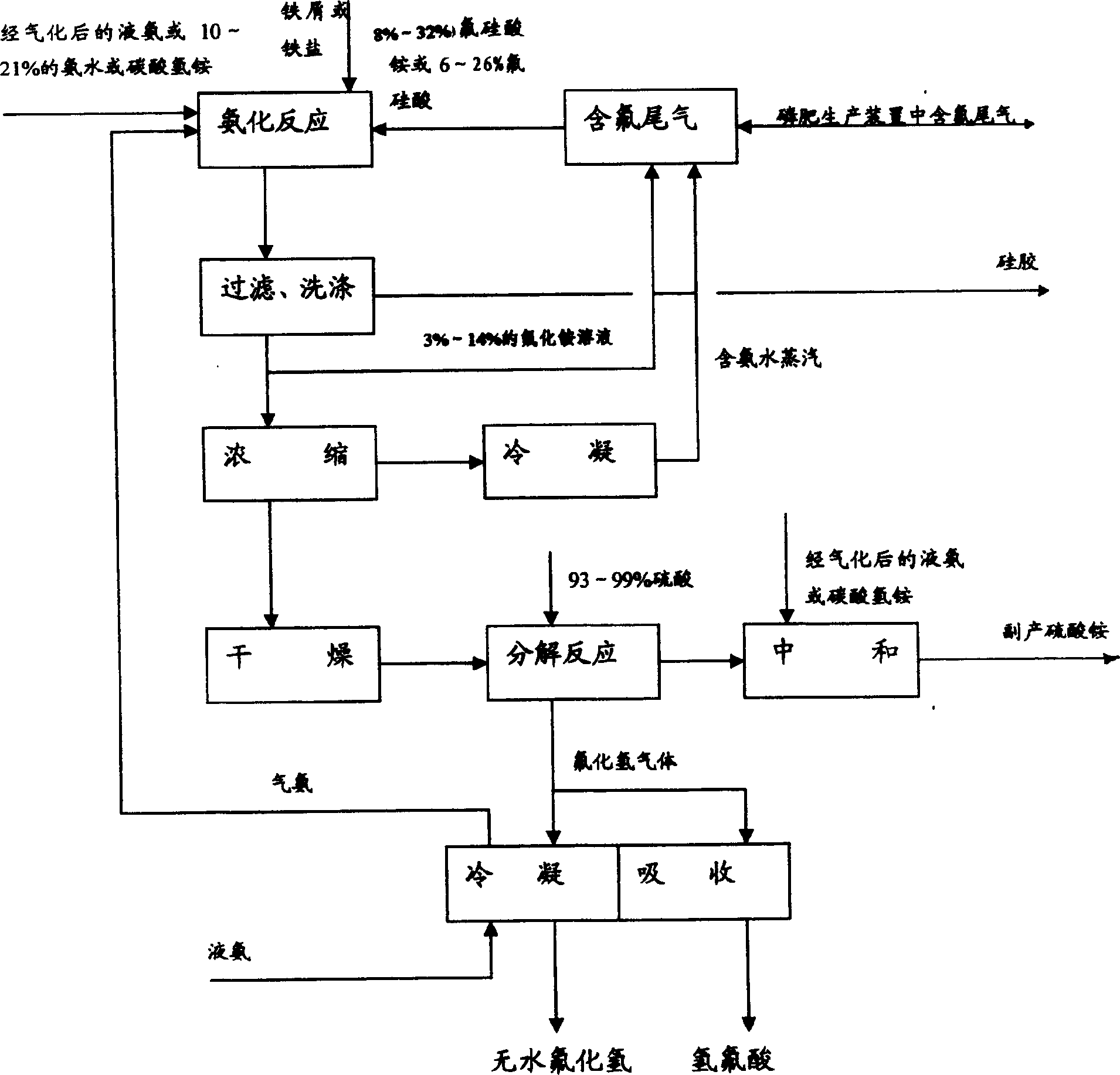
[0044] Absorb the by-product fluorine-containing tail gas of phosphate fertilizer production with 10% ammonium fluoride solution, obtain (NH 4 ) 2 SiF 6 22% ammonium fluorosilicate solution. Another by-product H in the ammonium fluorosilicate solution or phosphate fertilizer production 2 SiF 6 Add iron filings or iron salts (addition amount is 150% of theoretical amount) to fluosilicic acid with a content of 18%. The pH of the ammonium fluoride solution was controlled to be 8.5 after the ammoniation reaction. Filter, wash (twice) to remove silica gel. The entire ammonium fluoride solution was vacuum concentrated (85° C.) to a water content of <4%, and dried to obtain solid ammonium hydrogen fluoride. Solid ammonia hydrogen fluoride (98%) reacts with concentrated sulfuric acid (135% of the theoretical amount) at high temperature (140°C), and the generated HF gas is condensed or absorbed to produce anhydrous hydrogen fluoride and hydrofluoric acid. The mixture of ammonium...
Embodiment 2
[0077] The other processes are the same as in Example 1 except that the ammonia-containing water vapor produced by concentration is absorbed by fluosilicic acid and then returned to the system, or sent to the phosphate fertilizer system to absorb fluorine-containing tail gas after condensation, or sent to other ammonia-using devices for recovery.
Embodiment 3
[0079] In addition to neutralizing 18% fluorosilicic acid (P 2 o 5 content is 0.05%) solution, while adding iron filings or iron salt (addition amount is 200% of theoretical amount). After the ammoniation reaction, the pH of the ammonium fluoride solution was controlled to be 9.0. Filter, wash (twice) to remove silica gel. The entire ammonium fluoride solution was vacuum concentrated (at 85° C.) to a water content of <4%, and dried to obtain solid ammonium hydrogen fluoride. Solid ammonium hydrogen fluoride reacts with concentrated sulfuric acid (93%, the amount added is 100% of the theoretical amount) at high temperature (180°C); the mixture of ammonium sulfate and ammonium bisulfate is mixed with vaporized liquid ammonia. Other processes are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com