Binding domain-immunoglobulin fusion proteins
A technology of immunoglobulin, human immunoglobulin, application in binding domain-immunoglobulin fusion protein, treatment of malignant conditions and B cell diseases, single chain Fv-immunoglobulin fusion protein, molecularly engineered binding domain-immunoglobulin In the field of globulin fusion proteins, it can solve problems such as short half-life, loss of anti-tumor activity, and growth arrest
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
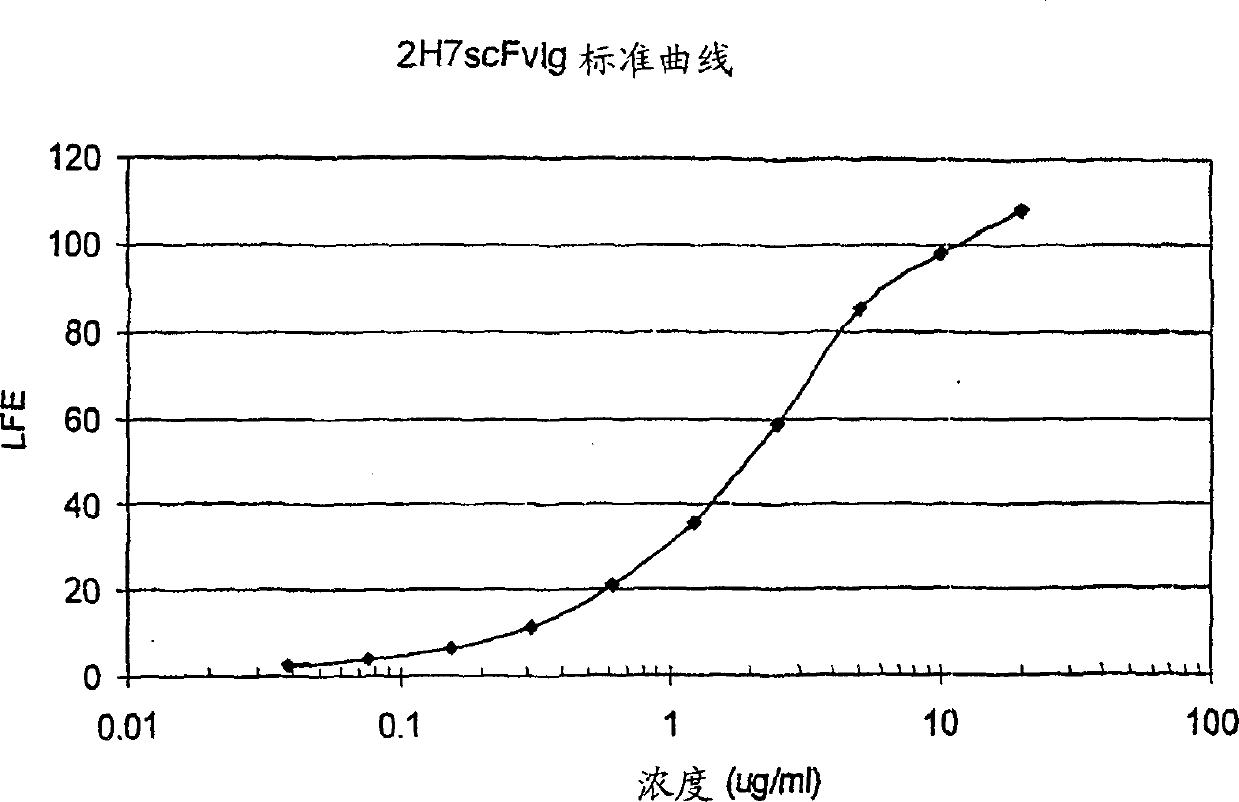
[0165] Cloning of 2H7 variable region and sequencing of 2H7SCFV-IG
[0166] This example illustrates the cloning of cDNA molecules encoding the heavy and light chain variable regions of monoclonal antibody 2H7. This example also demonstrates the construction, sequencing and expression of 2H7 scFv-Ig.
[0167] Hybridoma cells expressing the 2H7 monoclonal antibody specifically binding to CD20 were provided by Ed Clark, University of Washington, Seattle, WA. Prior to harvest, hybridoma cells were maintained in logarithmic phase in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with glutamine, pyruvate, DMEM non-essential amino acids, and penicillin-streptomycin. Resuspend the cells from the medium by centrifugation with 2 x 10 7 cells make RNA. RNA was isolated from 2H7-producing hybridoma cells using the Pharmingen (San Diego, CA) Total RNA Isolation Kit (Cat# 45520K) according to the manufacturer's instructions accompanying the kit. cDNA was prepared b...
Embodiment 2
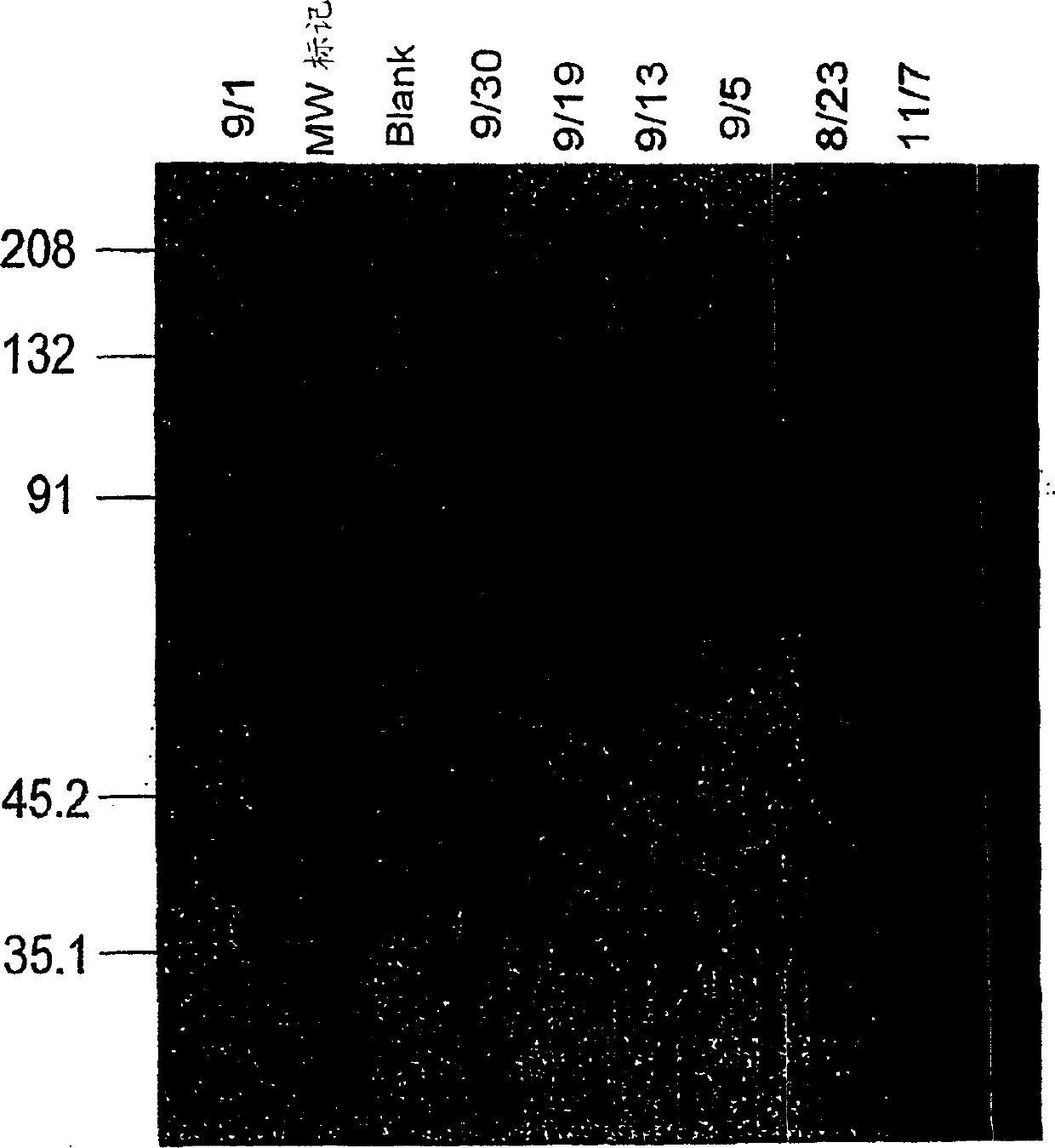
[0173] Expression of 2H7 SCFV-IG in Stable CHO Cell Lines
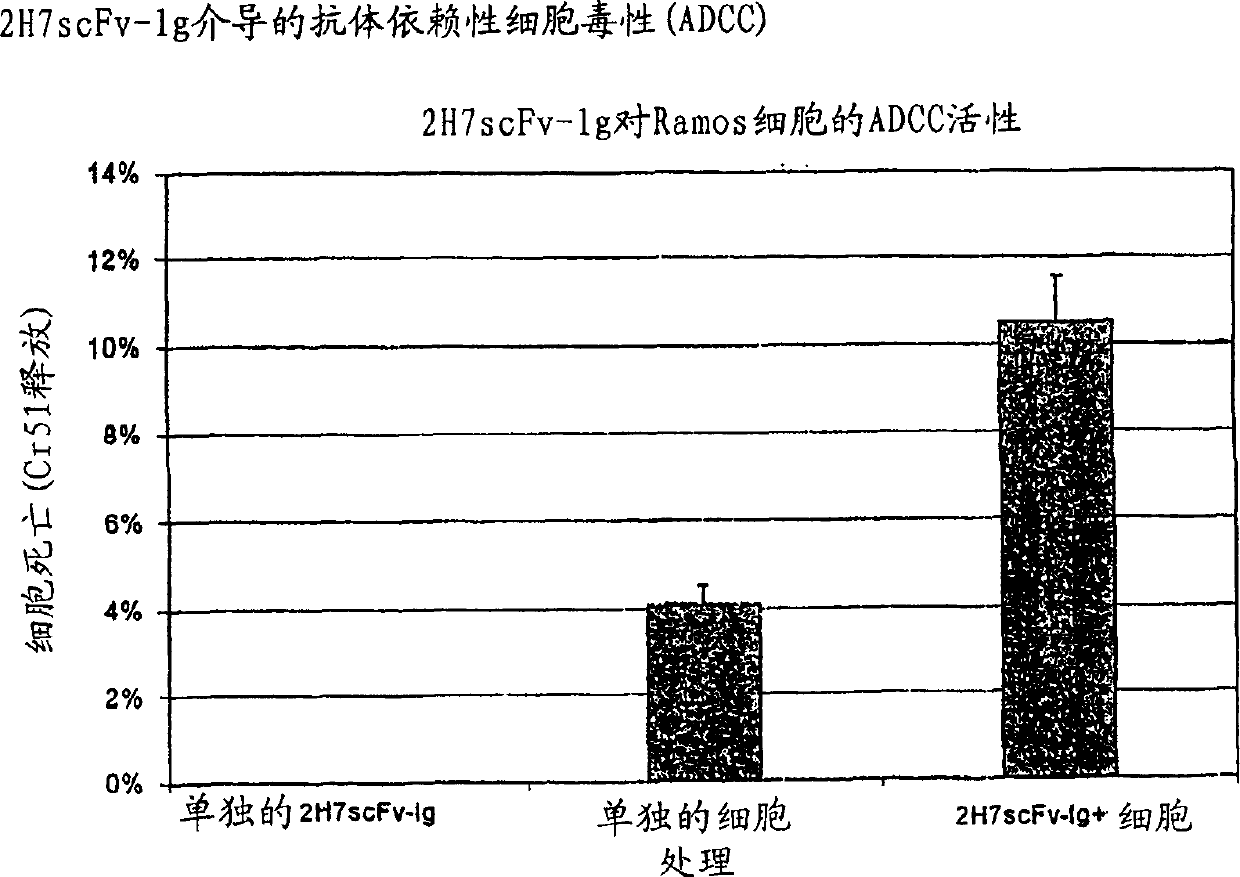
[0174] This example demonstrates the expression of 2H7 scFv-Ig in eukaryotic cells and the identification of expressed 2H7 scFv-Ig by SDS-PAGE and functional assays including ADCC and complement fixation.
[0175] 2H7scFv-Ig HindIII-XbaI (about 1.6 kb) with the correct sequence was inserted into mammalian expression vector pD18, and DNA of positive clones was amplified with QIAGEN therapeutic preparation kit (QIAGEN, Valencia, CA). Recombinant plasmid DNA (100 μg) was then linearized in non-essential regions by digestion with AscI, purified by phenol extraction, and resuspended in tissue culture medium Excell302 (Catalog No. 14312-79P, JRH Biosciences, Lenexa, KS). The cells used for transfection, CHO DG44 cells, were in logarithmic growth, and 10 cells were harvested for each transfection reaction. 7 cells. Linearized DNA was added to CHO in a total volume of 0.8 ml for electroporation.
[0176] Stable production ...
Embodiment 3
[0184] Effects of simultaneous ligation of CD20 and CD40 on normal B cell growth, as well as on CD95 expression and induction of apoptosis
[0185] This example illustrates the effect of cross-linking of cell surface expressed CD20 and CD40 on cell proliferation.
[0186] Dense resting B cells were isolated from human tonsils by a Percoll step gradient and T cells were depleted by E-rosetting. Through the last 12 hours of the 4-day experiment 3 [H]-thymidine uptake measured in dense tonsil B cells. Proliferation was measured in quadruplicate cultures, mean and standard deviation are indicated. Mouse anti-human CD20 mAb 1F5 (anti-CD20) alone or cross-linked with anti-mouse kappa mAb 187.1 (anti-CD20XL) was used. CD40 activation was accomplished using soluble human CD154 fused to murine CD8 (CD154) (Hollenbaugh et al., EMBO J 11:4212-21 (1992)), and CD40 crosslinking was accomplished using anti-mouse CD8 mAb 53-6 (CD154XL). This procedure allows simultaneous cross-linking of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com