Nux-vomica seed slow-released preparation and proless for preparing same
A technology of sustained-release preparations and Nuxe chinensis, applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve problems such as narrow safety range, achieve definite curative effect, simple preparation method, and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
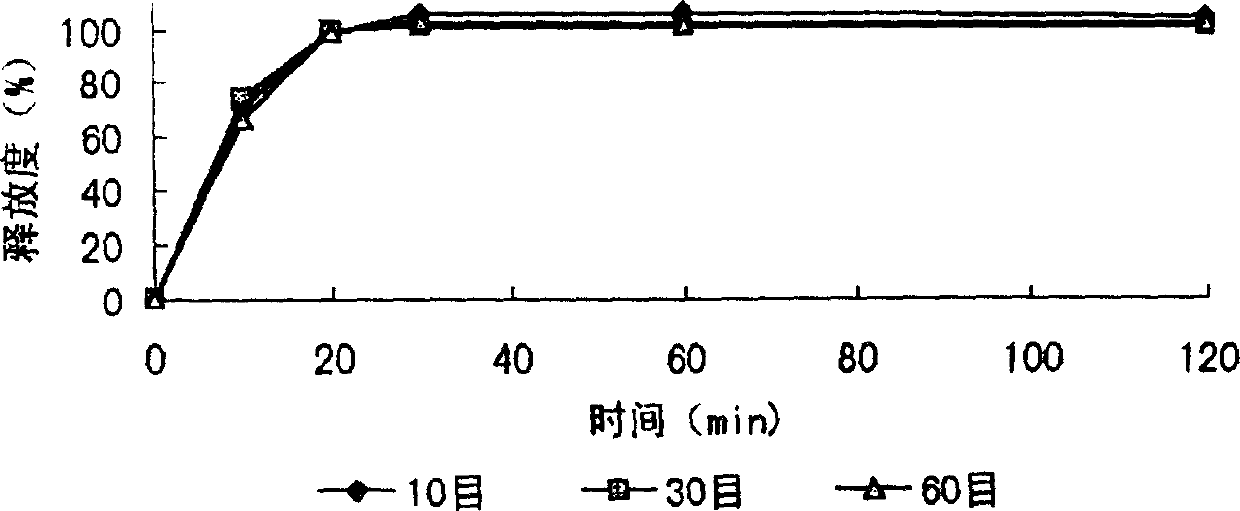
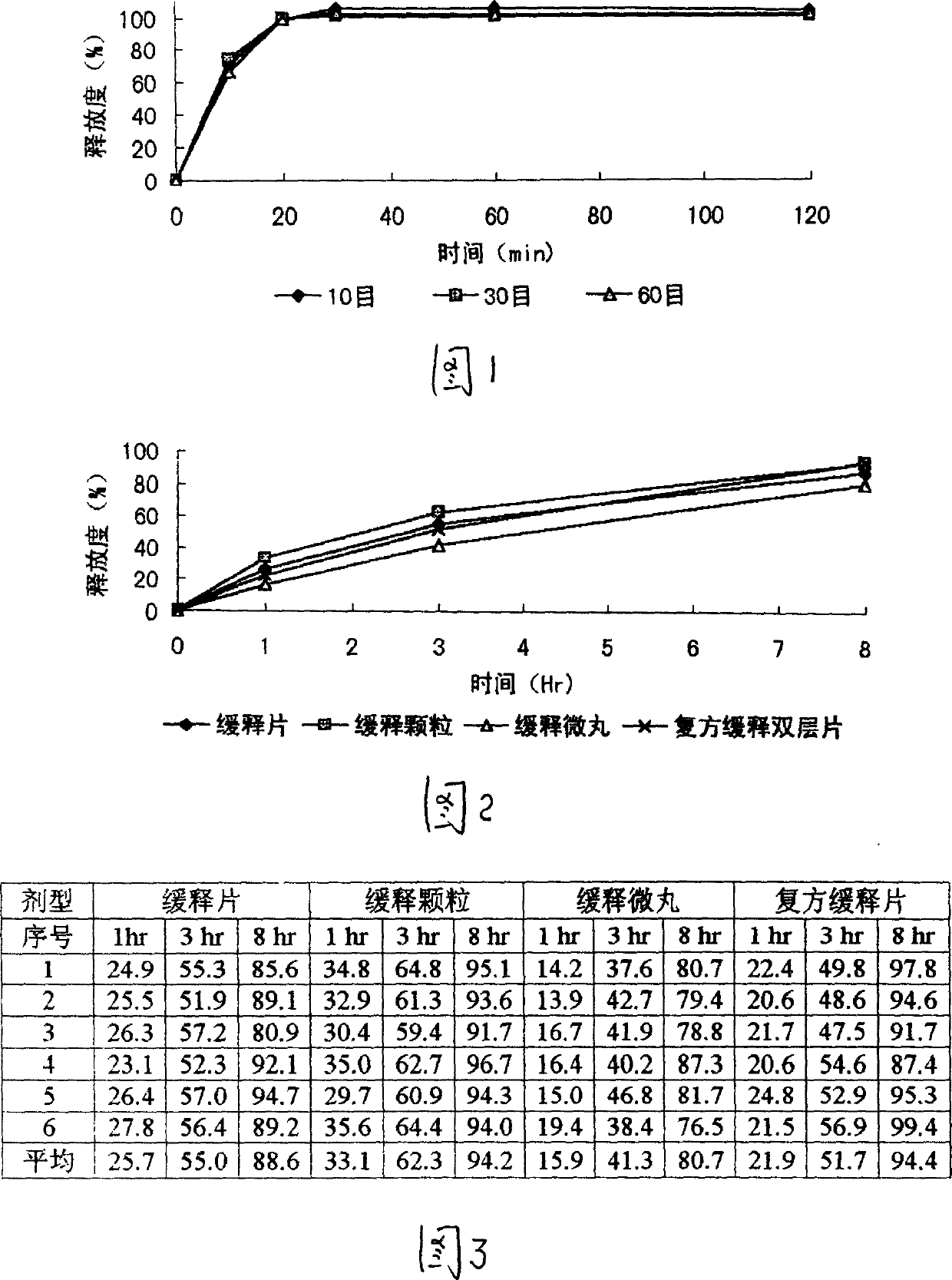
Image
Examples
example 1
[0034] Example 1, preparation of Nuxychnium sustained-release tablet
[0035] (1) Take the following raw materials
[0036] Appropriate amount of alkaloid extract (each tablet contains strychnine gmg)
[0037] Hypromellose 150g
[0038] Sodium Carboxymethyl Cellulose 50g
[0040] Lactose 50g
[0041] Stearic acid 30g
[0042] A total of 1000 pieces were made
[0043] (2) Production process
[0044] Grind Nuxychee into medium powder, add an appropriate amount of ammonia water, add 3 to 5 times the amount of chloroform, soak for 24 hours, and use a fat extractor to reflux extract for 3 to 5 hours; after the extract is concentrated to a suitable volume, add 3→ After extracting three times with 100% sulfuric acid, adjust the pH of the acid solution to 9-10, add chloroform for extraction three times, evaporate the chloroform to dryness, and obtain alkaloid powder; Mix blocker, diluent, etc., add wetting agent appropriate...
example 2
[0045] Example 2, preparation of Nuxychnium sustained-release granules
[0046] After the formula and technology of example 1 are used to make dry granules, granulate with 10 mesh and 14 mesh sieves, keep the part that can cross 10 mesh sieves and cannot cross 14 mesh sieves, after adding an appropriate amount of talcum powder, coating (coating material) 2% ethyl cellulose ethanol solution), the granules are prepared, packaged, and obtained. The finished product is tested for release rate, and the results are shown in the attached table.
[0047] The obtained slow-release granules can be mixed with corresponding quick-release granules prepared from other Chinese and Western medicine quick-release components to prepare compound sustained-release granules.
example 3
[0048] Example 3, preparation of nuxychnium sustained-release pellets and sustained-release capsules
[0049] (1) Take the following ball core raw materials
[0050] Alkaloid extract appropriate amount (each dose contains strychnine 8mg)
[0051] Hypromellose 50g
[0052] Starch 250g
[0053] (2) Take the following coating liquid raw materials
[0054] Ethyl cellulose 20 g
[0055] Polyethylene glycol 5 grams
[0056] Talc powder 50g
[0057] 95% ethanol 1000ml
[0058] Made a total of 1000 doses
[0059]The preparation method of sustained-release pellets: take an appropriate amount of extract powder (approximately equivalent to 8 grams of strychnine), add 20 milliliters of 95% ethanol, and ultrasonically dissolve it completely to obtain a spare solution; weigh 250 grams of starch, hydroxy Add 50 grams of propyl methylcellulose, after mixing evenly in equal amounts, use the spare solution as a wetting agent to make a soft material, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com