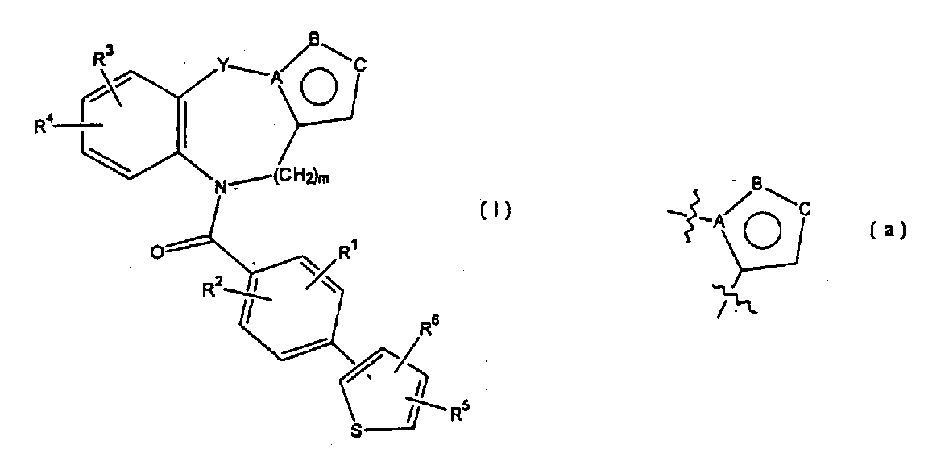
Thienylbenzoylbenzapines as vasporessin agonists
A technology of azepine and benzo, applied in the field of tricyclic aryl thiophene, can solve problems such as synthesis defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 (2-chloro-4-thiophen-2-yl-phenyl)-(5H-10,11-dihydro-pyrrolo[2,1-c][1,4]
[0067] Benzodiazepin-10-yl)-methylketone Step A. (4-Bromo-2-chlorophenyl)-(5H,11H-pyrrolo[2,1-c][1,4]benzene Diazepin-10-yl)-methyl ketone
[0068] N,N-Dimethylformamide (1 drop) was added to a solution of 4-bromo-2-chlorobenzoic acid (2.30 g) in anhydrous tetrahydrofuran (20 ml). Oxalyl chloride (1.46g) was added and the mixture was heated to reflux. The resulting solution was cooled to room temperature and then evaporated to dryness to give crude 4-bromo-2-chlorobenzoyl chloride as a golden viscous liquid which was used without further purification.
[0069] To 10,11-dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepine (1.44g) and triethylamine (0.95g) cooled in an ice bath To the mixture in methyl chloride (40ml) was added dropwise a solution of crude 4-bromo-2-chlorobenzoyl chloride (2.42g) in dichloromethane (20ml). The cooling bath was removed, and after...
Embodiment 2
[0076]Example 2 [2-chloro-4-(5-chlorothiophen-2-yl)-phenyl]-(5H,10,11-dihydropyrrolo[2,1-
[0077] c][1,4]benzodiazepin-10-yl)-methyl ketone
[0078] 5-Chlorothiophene-2-boronic acid (0.65 g, 4 mmol) was added to (4-bromo-2-chlorophenyl)-(5H,11H-pyrrolo[2,1-c][ 1,4] Benzodiazepin-10-yl)-methylketone (1.61g, 4mmol) and sodium carbonate (1.02g, 9.6mmol) in toluene (36ml), ethanol (10ml) and water (20ml) in the mixture. In the resulting solution, nitrogen was charged for 10 minutes, then tetrakis(triphenylphosphine)palladium(0) catalyst (0.18g, 0.16mmol) was added, the solution was heated to reflux for 41 hours, cooled to room temperature, filtered through diatomaceous earth, and then washed with Ethyl acetate rinse. The combined filtrates were diluted to 140 mL with water / ethyl acetate (1:1). The combined organic extracts were dried over anhydrous magnesium sulfate, filtered and evaporated to dryness. The residue (brown foam) was purified by flash chromatography on...
Embodiment 3
[0080] Example 3 [2-chloro-4-(5-methylthiophen-2-yl)-phenyl]-(5H,10,11-dihydropyrrolo[2,1-
[0081] c][1,4]benzodiazepin-10-yl)-methyl ketone
[0082] The title compound was prepared essentially according to the conditions set forth by Ohta et al., Heterocycles, 31, 1951 (1990). Potassium acetate (0.44 g, 4.5 mmol) was added to (4-bromo-2-chlorophenyl)-(5H,11H-pyrrolo[2 , 1-c][1,4]benzodiazepin-10-yl)-methyl ketone (1.2g, 3mmol) and 2-methylthiophene (1.5ml, 15.49mmol) N,N-di Methylacetamide (7.5ml) solution. The resulting solution was purged with nitrogen for 15 minutes, then tetrakis(triphenylphosphine)palladium(0) catalyst (0.17 g, 0.15 mmol) was added, and the sealed test tube was heated at 150° C. in an oil bath for 16.5 hours under vacuum The solvent was removed and the residue was triturated with water (10ml) and extracted with dichloromethane. The organic extracts were dried over anhydrous sodium sulfate, filtered and evaporated to give ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com