Bromine adulterated photocatalytic multicrystal material possessing photocatalytic performance under visible light
A crystalline material, photocatalytic technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of short life, limited visible light utilization, low photocatalytic activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
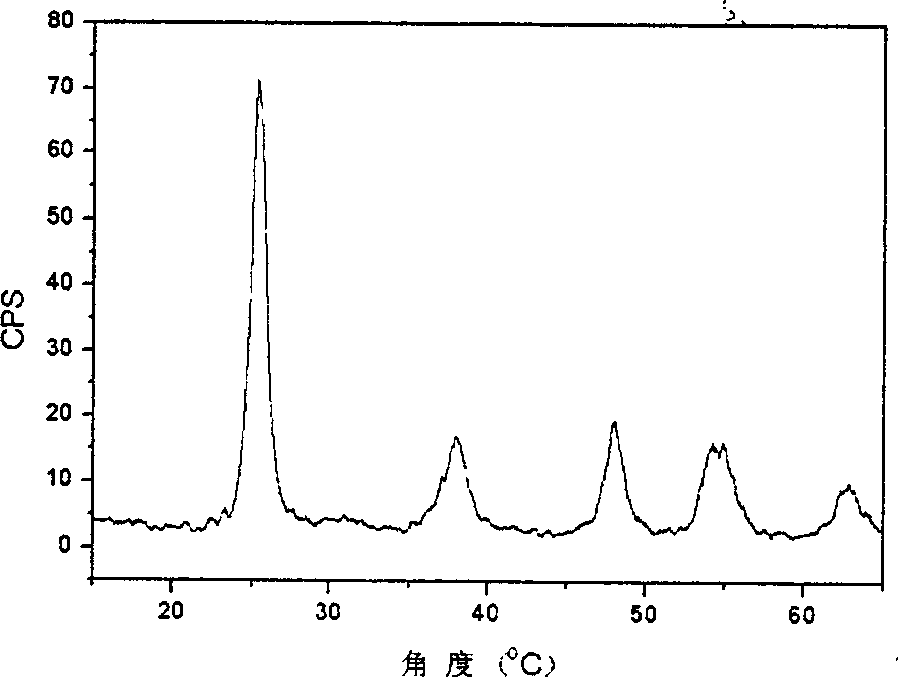
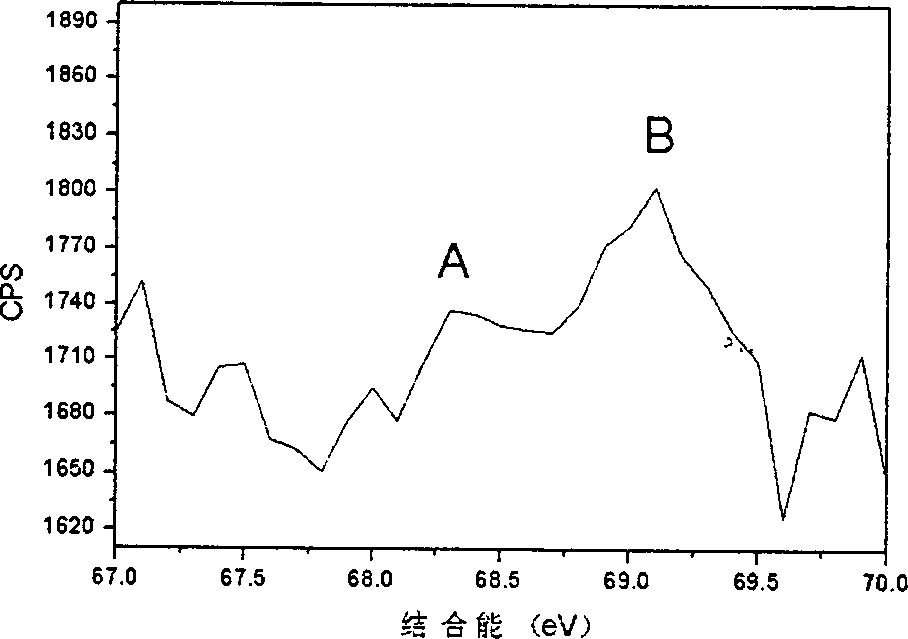
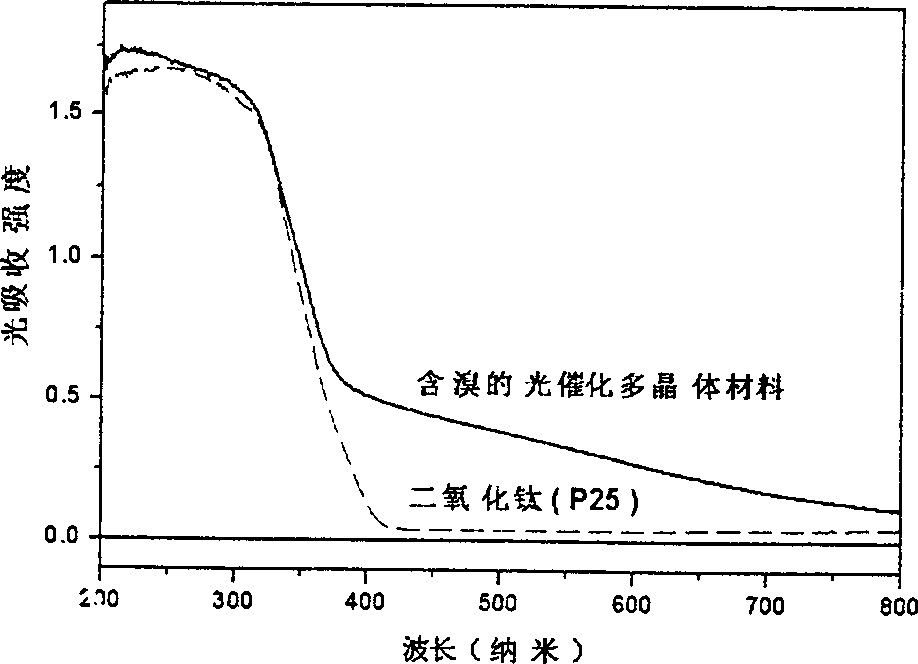
[0020] In the prepared bromine-doped photocatalytic polycrystalline material, the crystal phase is anatase phase; the content of titanium accounts for 54.55%, the content of oxygen accounts for 35.45%; the content of bromine accounts for 10.00%. Photocatalytic experiments prove that the photocatalytic activity of the bromine-doped photocatalytic polycrystalline material is 4 times that of titanium dioxide powder (P25 type) under visible light irradiation.
[0021] 30 ml of hydrogen bromide solution (2 mol / L) was reacted with 20 ml of tetrabutyl titanate (mass percentage>98.0%) until a white precipitate was completely formed. The white precipitate was directly heated in an oven, treated at 120° C. for 4 hours, evaporated to remove water and alcohols produced in the reaction to obtain a solid dry product. Then grind the obtained dry solid to make the particles uniform and reduce soft agglomeration. Put it into a muffle furnace after grinding, and calcinate at 200° C. for 2 hour...
Embodiment 2
[0023] In the prepared bromine-doped photocatalytic polycrystalline material, the crystal phases are anatase phase and crystal redite phase coexistence; the content of titanium accounts for 59.90%; the content of oxygen accounts for 40.00%; the content of bromine accounts for 0.10%. Photocatalytic experiments proved that the photocatalytic activity of the bromine-doped photocatalytic polycrystalline material was 5 times that of titanium dioxide powder (P25 type) under visible light irradiation.
[0024] 15ml of bromine aqueous solution (3% by mass) was reacted with 15ml of tetrabutyl titanate (>98.0% by mass) until a white precipitate was completely formed. The white precipitate was directly heated in an oven, treated at 120° C. for 4 hours, evaporated to remove water and alcohols produced in the reaction to obtain a solid dry product. Then grind the obtained dry solid to make the particles uniform and reduce soft agglomeration. Put it into a muffle furnace after grinding, an...
Embodiment 3
[0026] The prepared bromine-doped photocatalytic polycrystalline material has an anatase crystal phase; the content of titanium accounts for 59.78%, the content of oxygen accounts for 39.90%; the content of bromine accounts for 0.32%. Photocatalytic experiments prove that the photocatalytic activity of the bromine-doped photocatalytic polycrystalline material is 6 times that of titanium dioxide powder (P25 type) under visible light irradiation.
[0027] 15ml of hydrogen bromide solution (2mol / L) was reacted with 10ml of tetrabutyl titanate (mass percentage>98.0%) until white precipitate was completely formed. The white precipitate was directly heated in an oven, treated at 120° C. for 4 hours, evaporated to remove water and alcohols produced in the reaction to obtain a solid dry product. Then grind the obtained dry solid to make the particles uniform and reduce soft agglomeration. Put it into a muffle furnace after grinding, and calcinate at 300° C. for 2 hours to obtain a br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com