Preparation method of medical fiber with functions of reducing scar hyperplasia and easing pain
A scar hyperplasia and fiber technology, applied in the field of biomedical materials, to achieve the effects of increased stability, sustained and stable analgesic effect, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
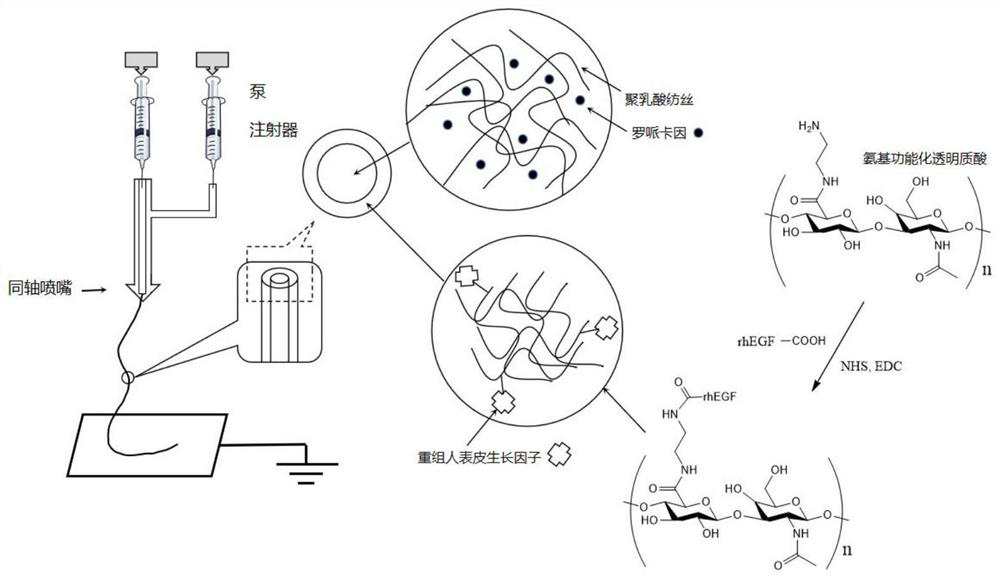
[0045] like figure 1 As shown, a medical fiber preparation method with functions of reducing scar hyperplasia and analgesia, comprising the following steps:
[0046] Step S1: Weigh 10 mg (1.60 nmol) of recombinant human epidermal growth factor (rhEGF for short), dissolve it in 2.0 ml pH 6.00 phosphate buffer solution, and add 15 mg (0.08 μmol) 1-(3-dimethylaminopropyl) -3-Ethylcarbodiimide hydrochloride (hereinafter referred to as EDC), and 9.2 mg (0.08 μmol) of N-hydroxysuccinimide (hereinafter referred to as NHS) in the above solution, ultrasonicated for 10 minutes, and then added 40 mg (0.8 nmol) of HA-NH2, placed in a shaker for 24 hours at room temperature, dialyzed with a 3Kd dialysis bag for 12 hours, and freeze-dried for later use.
[0047] Step S2: Dissolve 0.40g of gelatin and 0.10g of hyaluronic acid in a mixed solvent consisting of 2.25g of water and 2.25g of trifluoroethanol, sonicate until completely dissolved, and add 25 mg of the recombinant human epidermal gr...
Embodiment 2
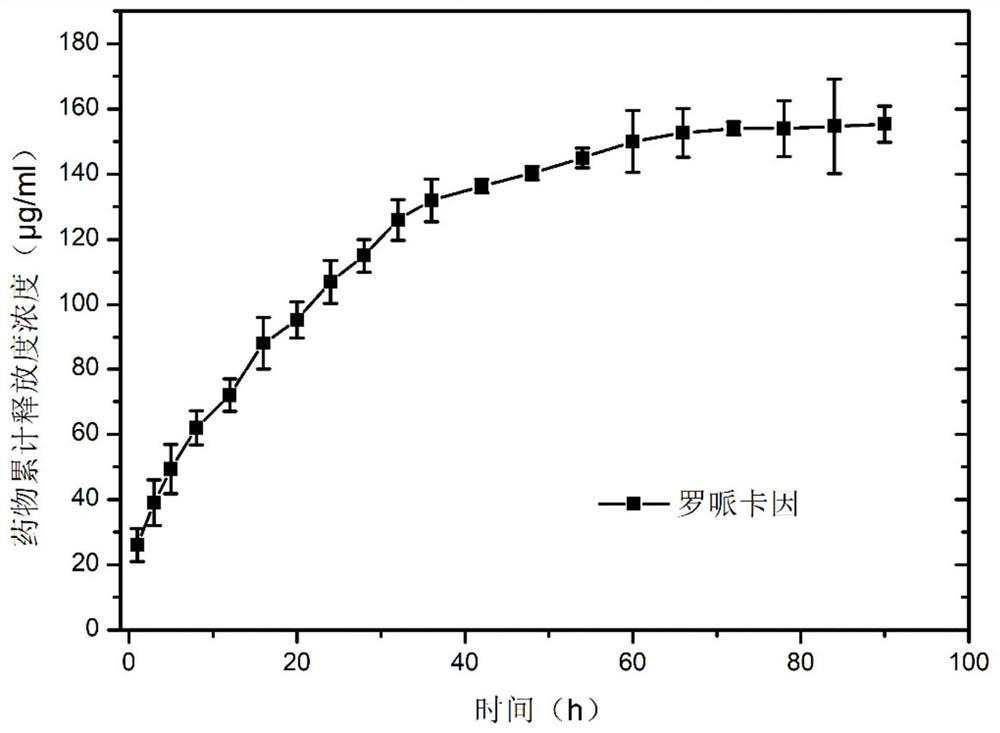
[0053] The medical fiber prepared in Example 1 was investigated in vitro drug release behavior:
[0054] Accurately weigh 50 mg of the medical fiber prepared in Example 1, put it into a centrifuge tube, add a PBS buffer solution (0.1 mol / L) with pH=7.40 as a release medium, shake to balance, place the centrifuge tube at 37 °C, shake Three replicates were performed in parallel on a shaking shaker with a frequency of 100 rpm. Draw the dissolution medium at a preset time point, add the same volume of release medium, measure the drug release concentration of ropivacaine with UV-Vis spectrophotometer, and calculate the cumulative release rate, the results are as follows figure 2 . Depend on figure 2 It can be seen that ropivacaine exhibits stable and continuous drug release, and does not reach the peak drug release until 72 hours, showing good sustained-release performance.
Embodiment 3
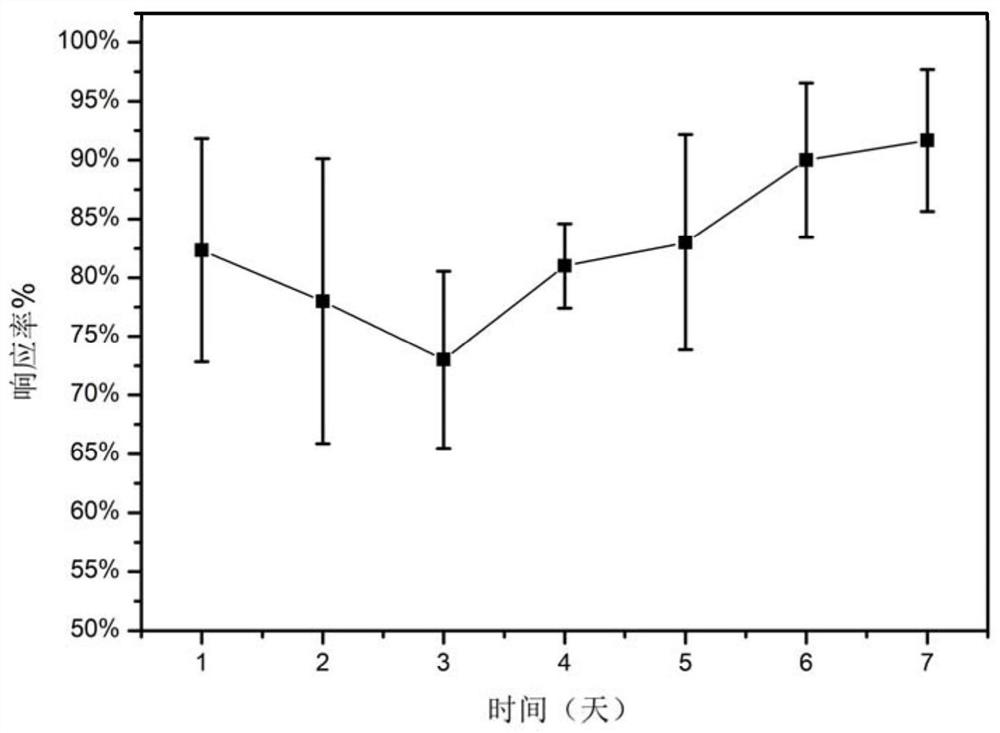
[0056] Anesthesia and analgesia experiment was performed on the medical fiber prepared in Example 1:
[0057] Select adult male rats weighing 300-350 g, make an incision of about 1 cm on the right back after anesthesia, implant spinning fibers and suture the wound. A clamping test was performed on the rats implanted with the medical fiber prepared in Example 1 to determine the analgesic effect of the drug. Specifically, brief squeezes were applied at six different locations within a 1 cm radius around the entire incision, and the animal's response to each stimulus was recorded. The normal response to stimulation is a withdrawal response or reflex contraction of the muscle tissue at the point of stimulation, while a negative response is seen as no withdrawal or muscle contraction. The response score is the percentage of times the animal responded to the stimulus as a percentage of the total number of stimuli. They were performed once a day for one week after the operation. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com