Compound medicine composition for treating glaucoma and application thereof
A composition and drug technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of lowering intraocular pressure, incompatibility, etc., and achieve the effect of reducing intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0070] Examples 1-5: Formulations Containing Netadil Dimesylate and Carteolol Mesylate
[0071] Table 1. Preparation of topical ophthalmic pharmaceutical composition solutions for lowering intraocular pressure and formulated as follows:
[0072]
[0073] The preparation process is as follows: 1) Weigh 95% of the water for injection of the recipe, add the active ingredient and auxiliary materials of the recipe and stir until completely dissolved, and adjust the pH to 4.5-5.4 with sodium hydroxide solution (10%); 2) Supplement water for injection To the full amount of the prescription, stir evenly and make the volume reach 100%; 3) Put the solution of step 2 into a low density polyethylene medicinal eye drop bottle, and study its stability under different storage temperature conditions.
[0074] Table 2. When stored at 5°C for 24 months, the content of each main drug component in Examples 1-5
[0075]
[0076] Table 3. Commercially available eye drops when stored at 5°C f...
Embodiment 6-10
[0089] According to Examples 1-5, the formulations containing NET and carteolol mesylate have good stability under the storage condition of 40°C for 14 days, which satisfies the requirements for commercially available eye drops. Short-term deviations from storage conditions. Examples 6-10: Formulations Containing Netandilol Dihydrochloride and Carteolol Hydrochloride
[0090] Table 8. Preparation of topical ophthalmic pharmaceutical composition solutions for lowering intraocular pressure and formulated as follows:
[0091]
[0092] Preparation process: Refer to the preparation process described in Examples 1-5.
[0093] Table 9. When stored at 5°C for 24 months, the content of each main ingredient in Examples 6-10
[0094]
[0095] According to Examples 6-10, the formulations containing NET and carteolol hydrochloride have good stability for 24 months under the storage condition of 5°C, which satisfies the requirements for commercially available eye drops. Requireme...
Embodiment 11
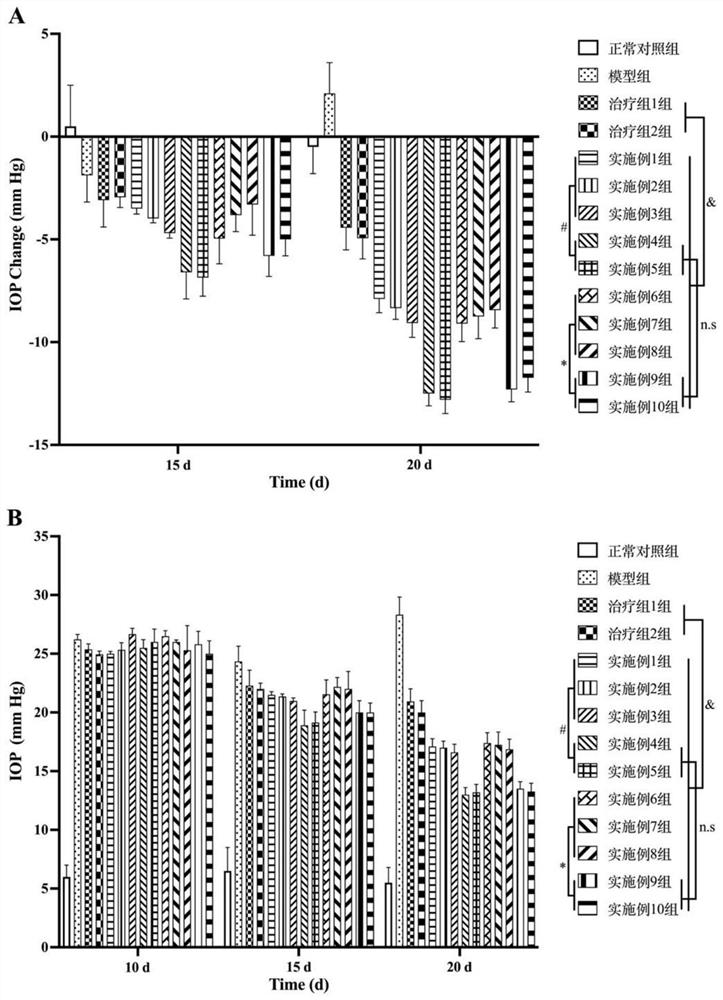
[0102] Example 11: Evaluating the efficacy of the ophthalmic pharmaceutical composition solutions of Examples 1-10 using the Japanese white rabbit glaucoma model
[0103] Experimental animals: healthy male Japanese white rabbits (Shenyang Pharmaceutical University, license number: SYXK (Liao) 2018-0009), with an initial weight of 2.5-3.0 kg, 3 rabbits / group.
[0104] Experimental group:
[0105] Normal control group: did not do any treatment.
[0106] Model group: establish a high intraocular pressure model without giving any eye drops.
[0107] Treatment group 1: establish a model of high intraocular pressure, and give commercially available netatadil eye drops Treatment group 2: established intraocular hypertension model, and given commercially available carteolol hydrochloride eye drops
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com