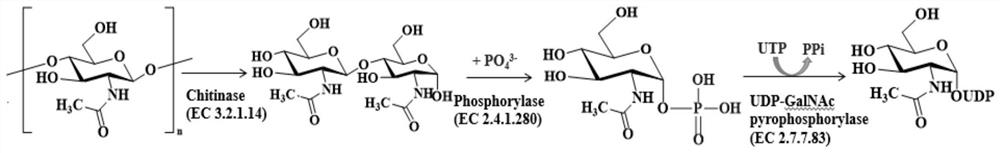
Double-enzyme co-immobilization synthesis method of uridine diphosphate-N-acetylglucosamine
A technology of uridine diphosphate and acetamido, applied in the field of biosynthesis, can solve the problems of inability to reuse enzymes, difficult to control intermediate products, low product yield, etc., and achieves improved pH tolerance and thermal stability, The effect of environmentally friendly process and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
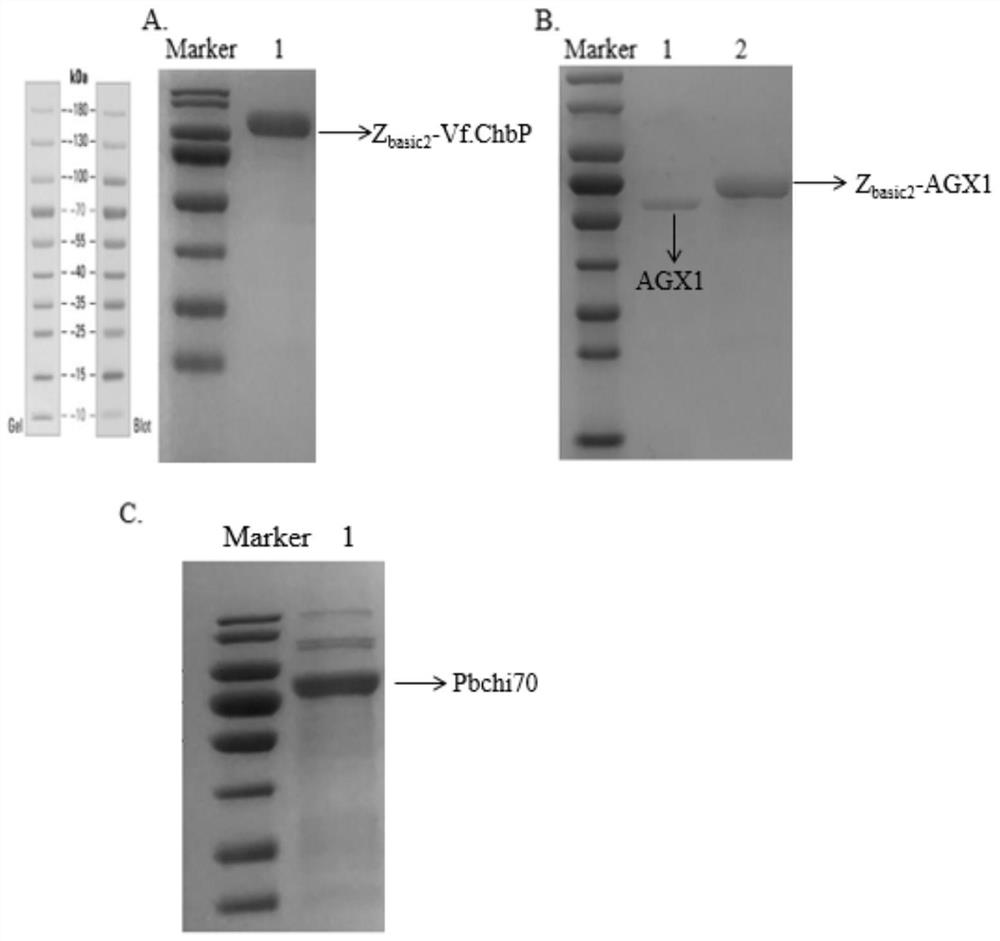
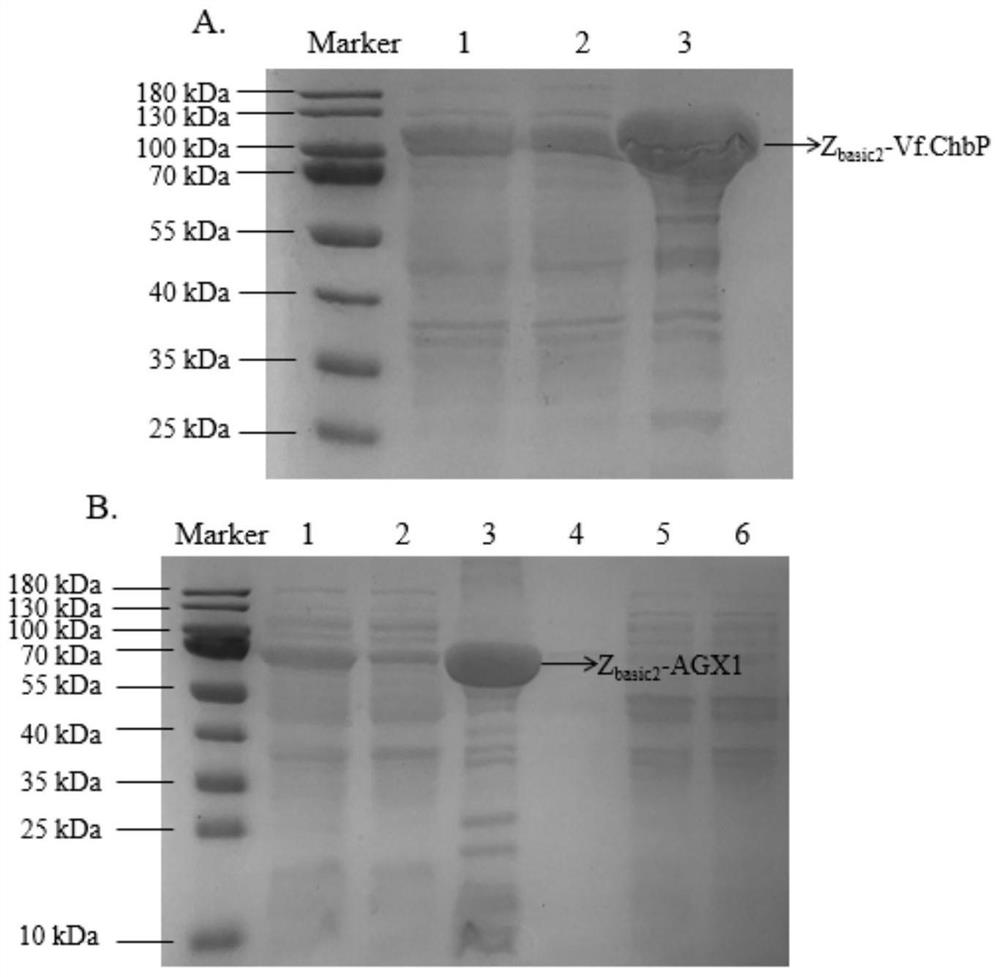
[0087] Example 1. Recombinant Escherichia coli PbChi70, Z basic2 -Vf.ChbP and Z basic2 -Expression of AGX1 with immobilization alone
[0088] 1. Using the chitinase gene PbChi70 and the phosphorylase gene Z respectively basic2 -Vf.ChbP and UDP-GalNAc pyrophosphorylase gene Z basic2 -AGX1 is the target gene, and pET-21a(+) is used as the vector plasmid to construct the recombinant plasmids PbChi70, Z basic2 -Vf.ChbP and Z basic2 -AGX1, then the recombinant plasmids PbChi70, Z basic2 -Vf.ChbP and Z basic2-AGX1 was transformed into Escherichia coli E.coliBL21(DE3) to obtain recombinant Escherichia coli containing chitinase gene PbChi70 and phosphorylase gene Z basic2 -Vf.ChbP recombinant E. coli and containing UDP-GalNAc pyrophosphorylase gene Z basic2 - AGX1 recombinant E. coli. The construction of the above recombinant bacteria was completed by Nanjing GenScript Biotechnology Co., Ltd.
[0089] The GenBank accession number of the chitinase gene PbChi70 is KJ634701.1. ...
Embodiment 2
[0098] Example 2. Catalytic reaction of chitinase PbChi70
[0099] Preparation of colloidal chitin: Weigh 4 g of chitin powder, slowly add it to 60 mL of concentrated hydrochloric acid, stir on ice, stir evenly, and place in a 4°C refrigerator overnight; add ice-cold 95% ethanol to the mixture, while Stir rapidly, at this time crystals are precipitated, centrifuge at 5000g for 20min at 4°C to collect the precipitate; rinse the precipitate repeatedly with double distilled water until the pH is 7.0; finally add 100 mL of double distilled water to prepare a 4% colloidal chitin solution.
[0100] Catalytic reaction: 250 μL of citric acid buffer (pH=5.5) and 500 μL of chitinase Pbchi70 purified in Example 1 were added to 250 μL of 4% (w / v) colloidal chitin, and reacted in a water bath at 55° C. for 1 h. After the reaction, the reaction solution was taken out, boiled for 5 min to terminate the reaction, centrifuged at 6000 rpm / min for 2 min, and the centrifuged supernatant was analy...
Embodiment 3
[0103] Example 3. Individually immobilized Z basic2 -Vf.ChbP immobilized preparation and Z basic2 - Catalytic reaction of AGX1 immobilized preparations
[0104] 1) Z basic2 - Determination of catalytic activity of Vf.ChbP immobilized preparations
[0105] Z prepared in Example 1 basic2 -Vf.ChbP immobilized preparation was added to the reaction system. The reaction system was shown in Table 3. After 1 h of reaction at 37°C and 1000 rpm / min, the reaction was terminated by boiling for 5 min, and centrifuged at 6000 rpm / min for 2 min at room temperature. TLC analysis was performed after filtration through a 0.45 μm filter.
[0106] Table 3 Individually immobilized enzyme Z basic2 - Catalytic reaction system of Vf.ChbP
[0107]
[0108] TLC results such as Figure 5 As shown, compared with the negative control, GlcNAc-1-P was produced in the reaction solution, indicating that Z basic2 -Vf.ChbP immobilized preparation has the activity of converting N,N-diacetylated chitob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com