Production process of tripeptide-29
A production process and intermediate technology, applied in the production process field of tripeptide-29, can solve the problems of large amount of organic solvent, waste of resources, high price, etc., and achieve the effect of reducing production cost, production cycle and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
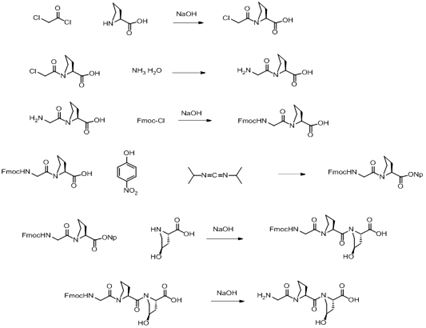
[0021] Step 1: Synthesis of Mca-Pro-OH (Intermediate-1)
[0022] In a 5L three-necked bottle, add 270g Pro, 540ml water, and 540ml toluene, keep the temperature at -5-0°C, drop a mixed solution of 292g chloroacetyl chloride and 146ml toluene into the feed liquid, and keep the pH of the system during the feeding and dropping process. At 8.5-9.0, after the reaction is detected by TLC, keep the temperature at 0-5 degrees, adjust the pH of the feed liquid to 2.0 ± 0.2, stir, filter with suction, wash the filter cake, and dry to obtain 414g Mca-Pro-OH, the yield 92.3%.
[0023] Step 2: Synthesis of H-Gly-Pro-OH (Intermediate-2)
[0024] In a 5L three-necked flask, add Mca-Pro-OH and 3L concentrated ammonia water, stir, and after TLC detects that the reaction is complete, the reaction solution is heated and concentrated to obtain H-Gly-Pro-OH.
[0025]Step 3: Synthesis of Fmoc-Gly-Pro-OH (Intermediate-3)
[0026] In a 5L three-necked bottle, add H-Gly-Pro-OH, 1242ml water, 1242ml...
Embodiment 2
[0034] Step 1: Synthesis of Mca-Pro-OH (Intermediate-1)
[0035] In the 5L three-necked bottle, add 200g Pro, 400ml water, 400ml ethyl acetate, keep the temperature at -5-0°C, add the mixed solution of 216g of chloroacetyl chloride and 108ml of ethyl acetate dropwise to the feed liquid, add materials and dropwise process The pH of the system was maintained at 8.5-9.0 in the middle, and after the reaction was detected by TLC, the temperature was maintained at 0-5 degrees, the pH of the feed liquid was adjusted to 2.0±0.2, stirred, suction filtered, and the filter cake was washed and dried to obtain 303 g of Mca-Pro- OH, yield 91.1%.
[0036] Step 2: Synthesis of H-Gly-Pro-OH (Intermediate-2)
[0037] In a 5L three-necked flask, add Mca-Pro-OH and 3L concentrated ammonia water, stir, and after TLC detects that the reaction is complete, the reaction solution is heated and concentrated to obtain H-Gly-Pro-OH.
[0038] Step 3: Synthesis of Fmoc-Gly-Pro-OH (Intermediate-3)
[003...
Embodiment 3
[0047] Step 1: Synthesis of Mca-Pro-OH (Intermediate-1)
[0048] In a 5L three-necked bottle, add 230g Pro, 460ml of water, and 460ml of acetonitrile, keep the temperature at -5-0°C, dropwise add a mixed solution of 249g of chloroacetyl chloride and 150ml of acetonitrile to the feed liquid, and keep the pH of the system during the addition and dropwise addition. At 8.5-9.0, after the reaction is detected by TLC, keep the temperature at 0-5 degrees, adjust the pH of the feed liquid to 2.0±0.2, stir, filter with suction, wash the filter cake, and dry to obtain 357g Mca-Pro-OH, the yield 93.3%.
[0049] Step 2: Synthesis of H-Gly-Pro-OH (Intermediate-2)
[0050] In a 5L three-necked flask, add Mca-Pro-OH and 3L concentrated ammonia water, stir, and after TLC detects that the reaction is complete, the reaction solution is heated and concentrated to obtain H-Gly-Pro-OH.
[0051] Step 3: Synthesis of Fmoc-Gly-Pro-OH (Intermediate-3)
[0052] In a 5L three-necked flask, add H-Gly-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com