Method for detecting N, N-dimethylformamide in ceftazidime residual solvent and application
A technology of dimethylformamide and ceftazidime, applied to N in the residual solvent of ceftazidime, can solve the problem of limited types of residual solvents, and achieve the effects of good system adaptability, good linearity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
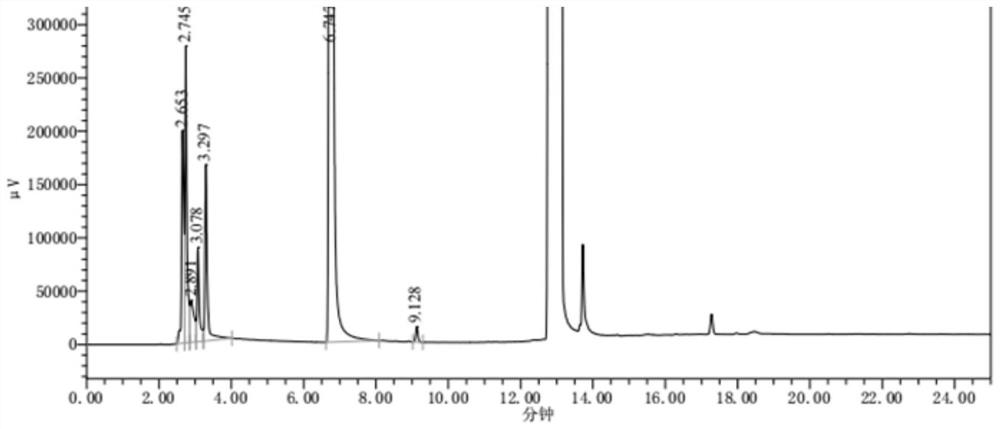
[0051] (1) Chromatographic conditions:
[0052] Instrument: high-efficiency gas chromatograph (Shimadzu GC-2014C), the detector is a hydrogen flame ionization detector (FID);
[0053] Chromatographic column: DB-WAXETR, 30m×0.53mm, 2.0μm or equivalent capillary column;
[0054] Column temperature: the initial temperature is 100°C, maintained for 3 minutes, and raised to 220°C at a rate of 10°C per minute, maintained for 10 minutes;
[0055] The inlet temperature is 230°C; the detector temperature is 250°C;
[0056] Injection volume: 1μL; column flow: 3.0mL / min;
[0057] Air flow: 400mL / min; hydrogen flow: 40mL / min; split ratio: 5:1.
[0058] (2) Solution preparation: the solution preparation process is shown in Table 1
[0059] Table 1 Solution preparation
[0060]
[0061] The specific operation is as follows:
[0062] Reference substance stock solution: Take 88mg of N,N-dimethylformamide, put it in a 10mL measuring bottle, add dimethyl sulfoxide to dissolve and dilut...
Embodiment 2
[0089] Verification Example 2: Linearity and Range
[0090] (1) Test process:
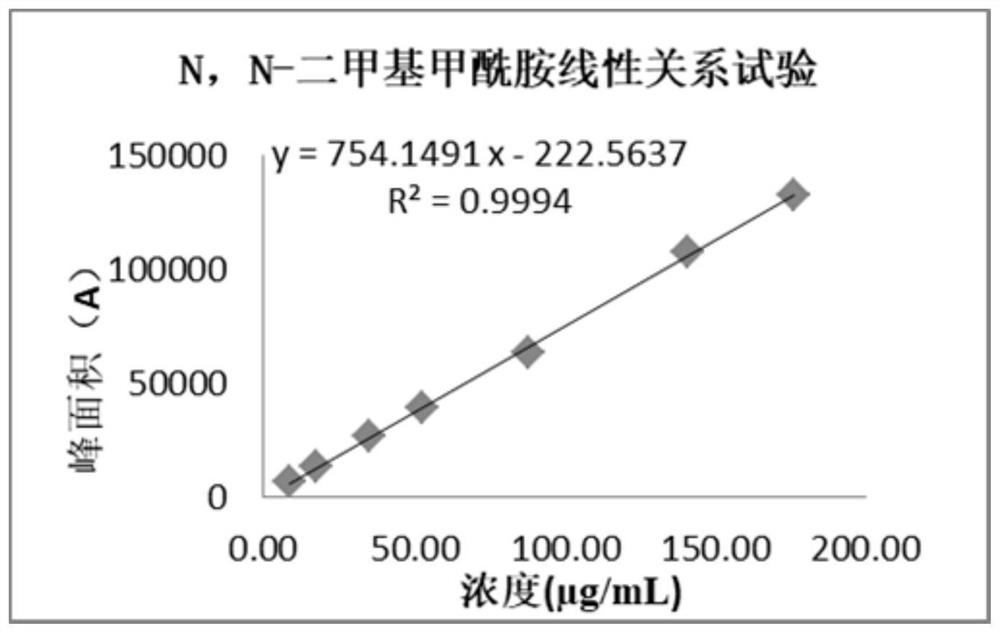
[0091] The linear solution preparation process is shown in Table 5 (N,N-dimethylformamide linear solution preparation process). Precisely measure 1 μL of each linear solution, inject them into the gas chromatograph, and record the chromatogram. The linear solution (10% linear solution) is the limit of quantification, as the starting point of linearity, the concentration of each solution is the abscissa, and the peak area of each solvent is the ordinate to carry out linear regression (results are shown in figure 2 ).
[0092] Table 5 N, N-dimethylformamide linear solution preparation process
[0093] linear solution Concentration (μg / mL) prepare 10% 8.80 Linear stock solution 1mL→20mL 20% 17.60 Linear stock solution 1mL→10mL 40% 35.20 Linear stock solution 2mL→10mL 60% 52.80 Linear stock solution 3mL→10mL 100% 88.00 Linear stock solution 5mL→10mL...
Embodiment 3
[0107] Validation Example 3: Limits of Detection and Limits of Quantitation
[0108] (1) Test process:
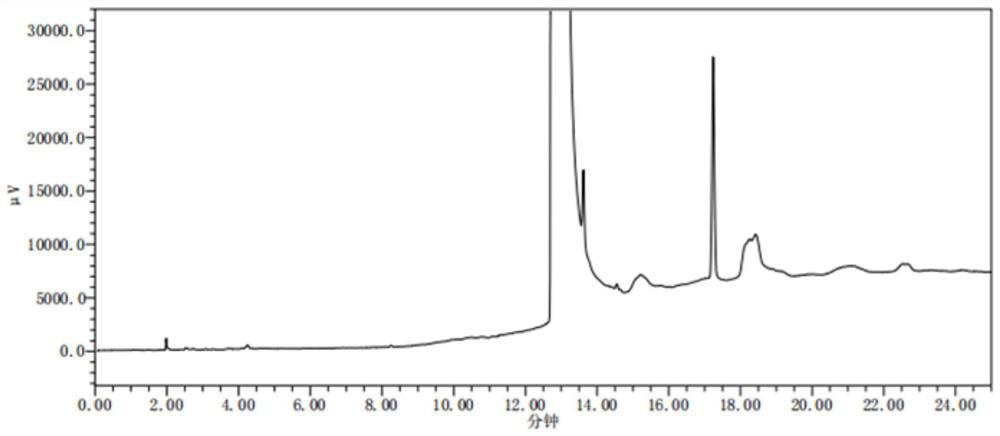
[0109] Accurately measure the linear solution (limit 10%) under "Verification Example 2: Linearity and Range", the signal-to-noise ratio (S / N) is greater than 10:1, which can be used as the limit of quantification. Dilute the limit of quantitation, according to the signal-to-noise ratio (S / N) greater than 3:1, can be used as the detection limit. The quantitative limit was injected 6 times continuously, the peak area was recorded, and the average value and RSD value were calculated.
[0110] (2) Results and conclusions:
[0111] Table 7 N, N-dimethylformamide quantitative limit and detection limit results
[0112]
[0113] Table 8 N, N-dimethylformamide quantitative limit system precision results
[0114] Element 1 2 3 4 5 6 average RSD(%) N,N-Dimethylformamide 6509 6810 6622 6545 6509 6491 6581 1.85
[0115] Table 9 N, N-dimethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com