Sinomenine derivative as well as preparation method and application thereof
A technology of sinomenine and derivatives, applied in the field of sinomenine derivatives and their preparation, can solve the problems of limited anti-fibrosis effect of sinomenine, and achieve good anti-fibrosis effect, good safety, and anti-fibrosis Effect of effect improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
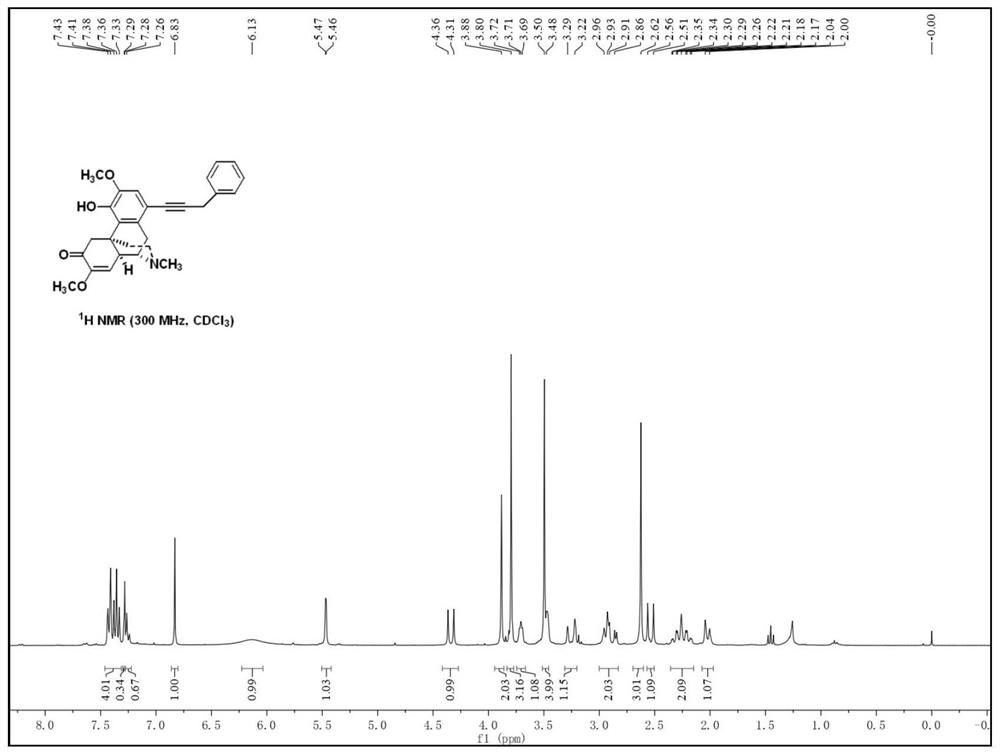
[0045] The preparation of embodiment 1 sinomenine derivatives
[0046]
[0047] The specific reaction steps for the preparation of the sinomenine derivatives are as follows:
[0048] S1. Compound (II) (20mmol, 1 equivalent) was dissolved in 160mL of dichloromethane, N-iodosuccinimide (21mmol, 1.05 equivalent) was added within 5 minutes, stirred at room temperature for 5 minutes, and 120mL Quenched with saturated sodium thiosulfate solution; extracted with dichloromethane, washed with brine, dried over magnesium sulfate, and evaporated under reduced pressure; the residue was purified by column chromatography to obtain compound (III) (yield: 86%);
[0049] S2, compound (III) (1mmol, 1 equivalent), 3-benzene-1-propyne (2mmol, 2 equivalents), ditriphenylphosphine palladium dichloride (0.05mmol, 0.05 equivalents), iodine Cuprous chloride (0.05mmol, 0.05eq), triethylamine (3mmol, 3eq) were dissolved in 10mL of anhydrous acetonitrile, stirred and reacted under argon protection fo...
experiment example 1
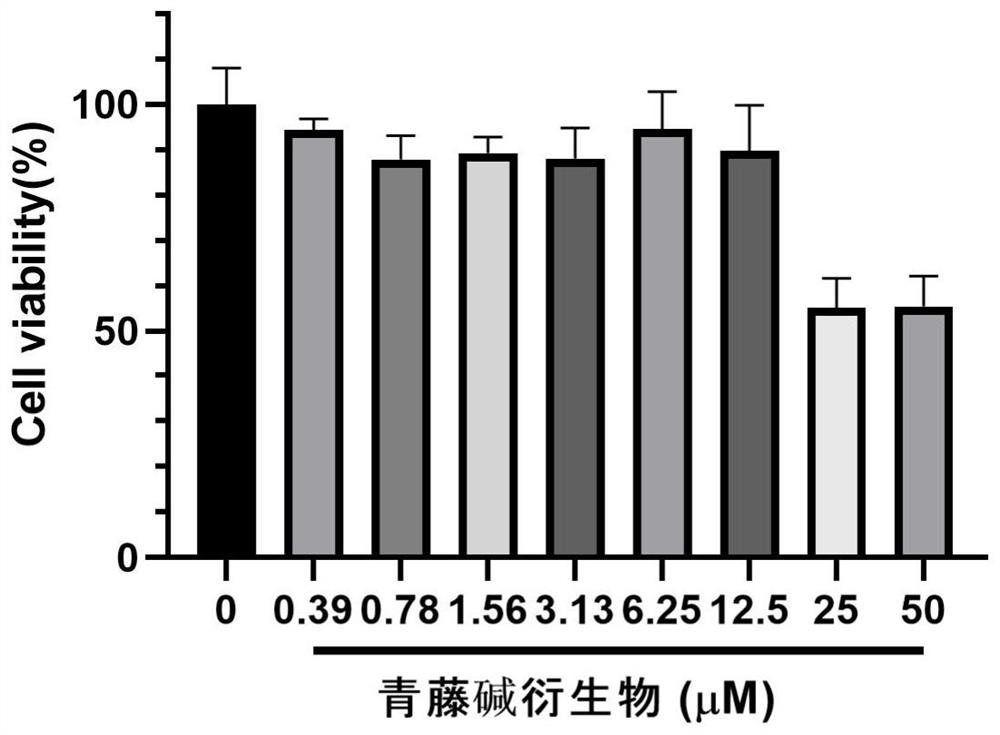
[0051] Experimental Example 1 Toxicity test (MTT method) of sinomenine derivatives to fibrotic cells
[0052] NIH / 3T3 cells (purchased from the Chinese Academy of Sciences) were placed in DMEM medium containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured in a humidified incubator. Add 100 μL of NIH / 3T3 cells at a concentration of 7000 / well to each well of a 96-well culture plate, at 37°C, 5% CO 2 Cultivate for 24 hours; Add 100 μL / well of two-fold gradient dilution of the test drug to the drug experimental group, 6 replicate wells for each concentration, set up a cell blank control and positive drug control pirfenidone (Pirfenidone) at the same time, and continue to cultivate for 24 hours; After adding 10 μl of MTT and continuing to incubate for 4 h, the supernatant was discarded, 150 μl of DMSO was added, and the absorbance at 490 nm was detected with a full-wavelength multifunctional microplate reader. The toxic effect of compounds on fibrotic cells was determined.
...
experiment example 2
[0054] Experimental example 2 Inhibitory effect of sinomenine derivatives on TGF-β1-induced fibrosis target mRNA in NIH / 3T3 cells
[0055] NIH / 3T3 cells in the logarithmic growth phase (purchased from the Chinese Academy of Sciences) were selected to make a single cell suspension and seeded on a culture plate; the cells were stimulated with 5 ng / ml TGF-β1 for 24 hours to construct a fibrotic cell model. Divided into normal group, model group (TGF-β1 group), positive drug group (Pirfenidone group), sinomenine derivatives group (0.78, 1.56, 3.13, 6.25, 12.5μM), sinomenine group ( 0.78, 1.56, 3.13, 6.25, 12.5 μM).
[0056] Cells were collected, and total cellular RNA was extracted with an RNA extraction kit, reverse-transcribed into cDNA with an RNA reverse transcription kit, and PCR amplified using a SYBR Premix Ex TaqTM11 kit. The above experiments were performed in accordance with the instructions. The primers were synthesized by Sangon Biotechnology Co., Ltd., and the seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com