Method for preparing copper-iron alloy based on electro-deposition method
A technology of copper-iron alloy and electrodeposition method, which is applied in the field of electrochemical deposition, can solve the problems of large difference in properties between copper and iron, high degree of alloy segregation, high impurity content, etc., achieve low cost, uniform coating thickness, and reduce hydrolysis reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] An embodiment of the present invention provides a method for preparing a copper-iron alloy by electrodeposition, comprising:
[0037] The sulfate aqueous solution system is used as the electrolyte, the copper plated or stainless steel plate is used as the negative electrode, the oxygen evolution anode is used as the positive electrode, and the pulse power supply is used for deposition. The low potential range is -3~-5V, and the high potential range is -3.5. ~-5.5V; pulse voltage is applied, and the corresponding cathode current density is 80~200mA / cm 2 , the pulse width range is 1-100ms, the electrolyte circulation rate is controlled at 700-1400mL / min, the pH value is 2.5-4, the deposition temperature is 45-50°C, and the deposition time is 15-120min; the distance between positive and negative electrodes is 1- 4cm, the obtained copper-iron alloy composition is Fe0.84-86wt%, Cu5.10-95wt%.
[0038] Wherein, the sulfate aqueous solution system electrolyte comprises the com...
Embodiment 1
[0043] (1) Taking the oxygen-analysis anode as the positive pole, and copper plating on the stainless steel plate as the negative pole, the sulfate aqueous electrolyte:
[0044] Fe(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O 200g / L; copper sulfate 80g / L; boric acid 20g / L; o-benzoylsulfonimide 3g / L; sodium gluconate 100g / L.
[0045] Using pulse power deposition, the potential range of low potential is -3.5V, and the potential range of high potential is -4V; pulse voltage is applied, and the corresponding cathode current density is 120mA / cm 2 , the pulse width range is 80ms, the electrolyte circulation rate is controlled at 1000mL / min, the pH value is 3, the deposition temperature is 50°C; the positive and negative electrode spacing is 3cm, and the electrodeposition is 30min to produce iron-copper alloy.
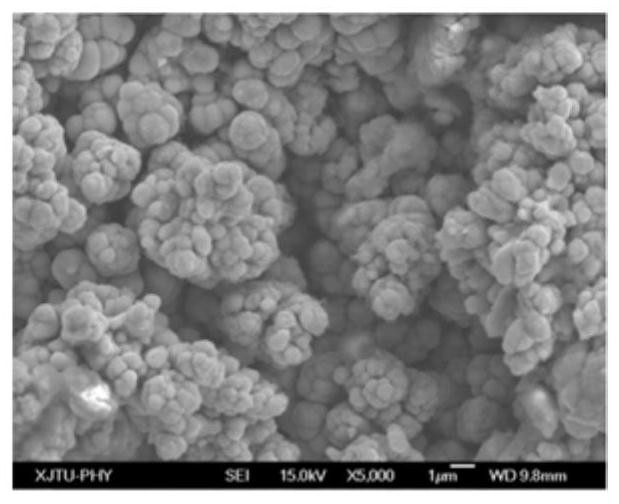
[0046] Using the electrochemical deposition method of this embodiment, an iron-copper alloy with a composition of 9.97wt% Fe, 87.24wt% Cu, uniform coating and low impurity content was con...
Embodiment 2
[0048] The oxygen-evolving anode is used as the positive electrode, the copper plating on the stainless steel plate is used as the negative electrode, and the sulfate aqueous electrolyte is used:
[0049] Fe(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O 300g / L; copper sulfate 30g / L; boric acid 20g / L; o-benzoylsulfonimide 5g / L; sodium citrate 80g / L.
[0050] Using pulse power deposition, the low potential potential is -3V, the high potential potential range is -3.8V; pulse voltage is applied, and the corresponding cathode current density is 80mA / cm 2 , the pulse width range is 1ms, the electrolyte circulation rate is controlled at 1200mL / min, the pH value is 2.5, the deposition temperature is 45°C; the positive and negative electrode spacing is 1cm, and the electrodeposition is 30min to produce iron-copper alloy.
[0051] The copper-iron alloy with the composition of 20.16wt% Fe, 76.38wt% Cu, uniform coating and low impurity content was continuously prepared by using the electrochemical depo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com