Ordered mesoporous copper-rare earth metal-aluminum composite oxide catalyst and application thereof
A technology of rare earth metals and oxides, applied in the field of ordered mesoporous copper-rare earth metal-aluminum composite oxide catalysts and its applications, can solve the problems of complex reaction process, decreased catalytic activity, high requirements, etc., and achieve selectivity and The effect of high yield, close interaction and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] At room temperature, 2.0g P123(EO 20 PO 70 EO 20 ) (0.00035mol) was dissolved in 40mL absolute ethanol, then added 3.2mL 35wt% hydrochloric acid (0.036mol), 0.286g salicylic acid (0.0016mol) and 0.2g citric acid (0.0010mol) and stirred vigorously, then added 4.08 g aluminum isopropoxide (0.02mol), 0.0857g copper acetate (0.00047mol) and 0.1616g lanthanum acetate (0.00051mol), vigorously stirred for 6h to obtain a uniform mixed solution. The solvent was evaporated at 60°C for 24h under an air atmosphere, then dried at 65°C for 48h. Finally, the obtained dried sample was put into a muffle furnace, and the temperature was raised to 400°C at a rate of 1°C / min for 4 hours, and then the temperature was continued to be raised to 700°C for 1 hour at a rate of 10°C / min, and the temperature was naturally lowered to obtain catalyst A.
[0036] Put the above-mentioned catalyst into the fixed-bed reactor, close the path connecting the hydrogen cylinder 1 to the reaction tube 10, ...
Embodiment 2
[0038] The preparation method of catalyst B is the same as that of Example 1, but the addition of copper acetate is 0.1429g (0.00079mol).
[0039] The reduction of catalyst B and the reaction method for producing higher alcohols from ethanol are the same as in Example 1.
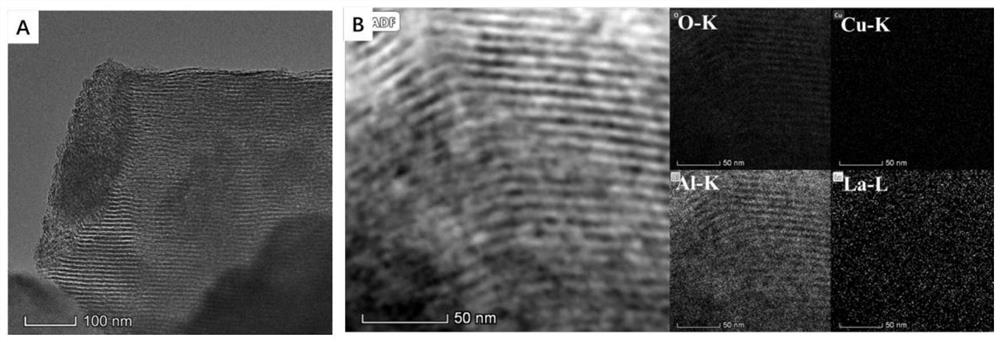
[0040] From figure 2 (A) The ordered mesoporous structure of Catalyst B after the reaction can be clearly seen [Chem Eng J., 2017, 303, 1583-1592.], and no discernible Cu nanoparticles were found. Distribute images in elements figure 2 In (B), the bright stripes correspond to the Al and O signals and clearly form an ordered framework, while the Cu-K and La-L signals are uniformly distributed on the ordered framework material. The above results indicate that the active components of copper and rare earth metal oxides are highly dispersed in the catalyst and exist stably due to the strong interaction among the three oxides of copper, lanthanum and aluminum.
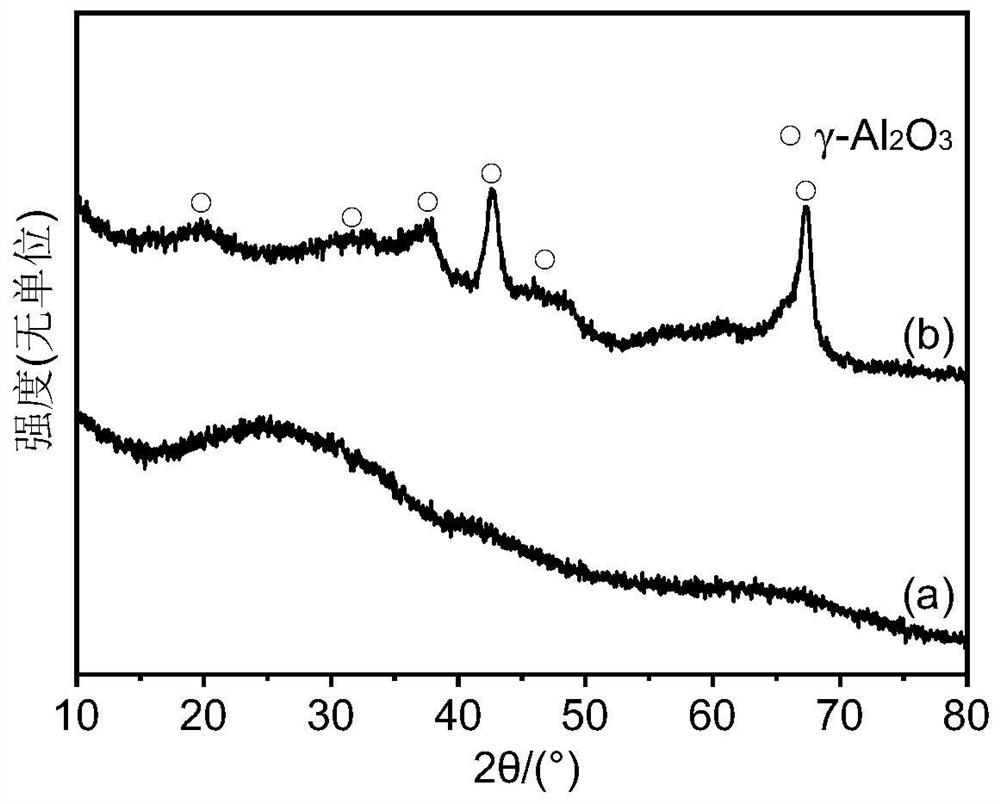
[0041] image 3 It is the XRD pattern of cata...
Embodiment 3
[0044] The preparation method of catalyst C is the same as in Example 1, but the copper acetate add-on is 0.1858g (0.00102mol).
[0045] The reduction of catalyst C and the reaction method for producing higher alcohols from ethanol are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com