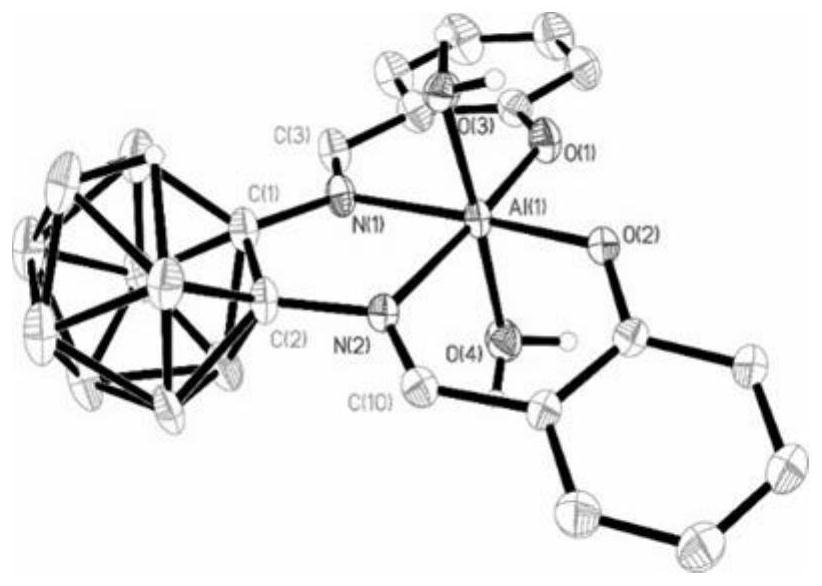
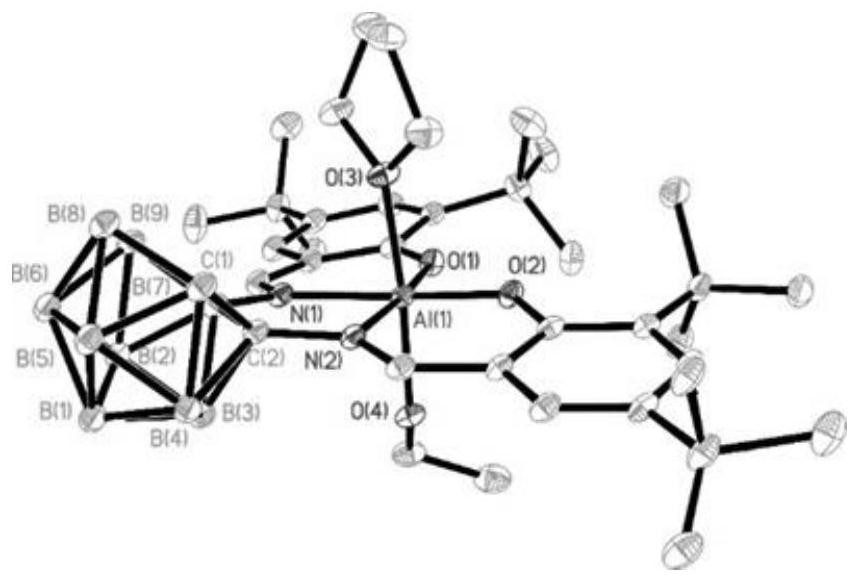
Carborane-supported salen ligand, nested carborane-supported salen-Al catalyst and preparation and application thereof
A carborane and ligand technology, which is applied to carborane-supported salen ligands, nested carborane-supported salen-Al catalysts, and their preparation and application fields, achieving industrialization, a simple synthesis process, and high selectivity Effects of Sex and Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of Ligand L1
[0045] Diaminocarborane (1.85g) and salicylaldehyde (3.24g) were added to a 100ml round-bottomed flask, 60ml of toluene was added to dissolve it, and then 0.8ml of trifluoroacetic acid was added. The lower end of the branch port of the water separator, the other end of the water separator is connected to a reflux condenser, heated with an oil bath at 130 ° C until the reaction system refluxes, the oil bath is removed after the reaction for 6 hours, and the excess solvent is removed by rotary evaporation. The obtained black solid is recrystallized with ethanol and filtered to obtain Yellow solid (2.80 g, yield: 70%).
[0046]
[0047] 1HNMR (400.13MHz, CDCl3): δ11.15 (s, 2H, OH), 8.71 (s, 2H, N=CH), 7.47–7.42 (m, 4H, HPh), 7.03–6.96 (m, 4H, HPh) );
[0048] 13C{1H}NMR (100.61MHz, CDCl3): δ170.96(N=CH), 161.26(CPh), 135.57(CPh), 133.91(CPh), 119.92(CPh), 117.94(CPh), 117.81(CPh) ,93.43(Co-carb);
[0049] 11B{1H}NMR (160.46MHz, DM...
Embodiment 2
[0051] Example 2: Synthesis of Ligand L2
[0052]In a 100ml round-bottomed flask, add diaminocarborane (1.00g) and tripenta-di-tert-butylsalicylaldehyde (3.00g), add 60ml of toluene to dissolve, and then add 0.8ml of trifluoroacetic acid, The bottle mouth of the round-bottomed flask is connected to the lower end of the branch port of the water separator, and the other end of the water separator is connected to a reflux condenser, heated with an oil bath at 135 ° C until the reaction system refluxes, and the oil bath is removed after the reaction for 6 h, and the excess solvent is removed by rotary evaporation. Recrystallization from ethanol and filtration gave a yellow solid (2.70 g, yield: 77%).
[0053]
[0054] 1HNMR (400.13MHz, DMSO-d6): δ11.62(s, 2H, OH), 8.70(s, 2H, N=CH), 7.50(s, 2H, HPh), 7.20(s, 2H, HPh), 1.38(s,18H,tBu),1.32(s,18H,tBu);
[0055] 13C{1H}NMR (100.61MHz, CDCl3): δ171.72(N=CH), 158.66(CPh), 141.47(CPh), 137.72(CPh), 130.54(CPh), 128.14(CPh), 117.14(...
Embodiment 3
[0058] Example 3: Synthesis of Ligand L3
[0059] Diaminocarborane (2.37g) and tetramethoxysalicylaldehyde (4.76g) were added to a 100ml round-bottomed flask, 60ml of toluene was added to dissolve, and 0.8ml of trifluoroacetic acid was added. The mouth of the flask is connected to the lower end of the branch port of the water separator, and the other end of the water separator is connected to a reflux condenser, heated with an oil bath at 125 ° C until the reaction system refluxes, and the oil bath is removed after the reaction for 6 hours, and the excess solvent is removed by rotary evaporation. The obtained black solid is reconstituted with methanol. Crystallized and filtered to give a yellow solid (3.70 g, yield: 62%).
[0060]
[0061] 1HNMR (400.13MHz, DMSO-d6): δ11.55 (s, 2H, OH), 8.52 (s, 2H, N=CH), 7.22 (s, 2H, HPh), 6.49-6.44 (m, 4H, HPh) ),3.79(s,6H,OMe);
[0062] 13C{1H}NMR (100.61MHz, CDCl3): δ169.47(N=CH), 165.69(CPh), 163.81(CPh), 135.21(CPh), 111.89(CPh), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com