1,2,4-oxadiazole-5-imine derivative and synthesis method thereof
A technology for oxadiazoles and derivatives, which is applied in the field of 1,2,4-oxadiazole-5-imine derivatives and their synthesis, can solve the problems of cumbersome post-processing, rare products containing chiral structures, and limitations Application range and other issues, to achieve the effect of high selectivity and yield, good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
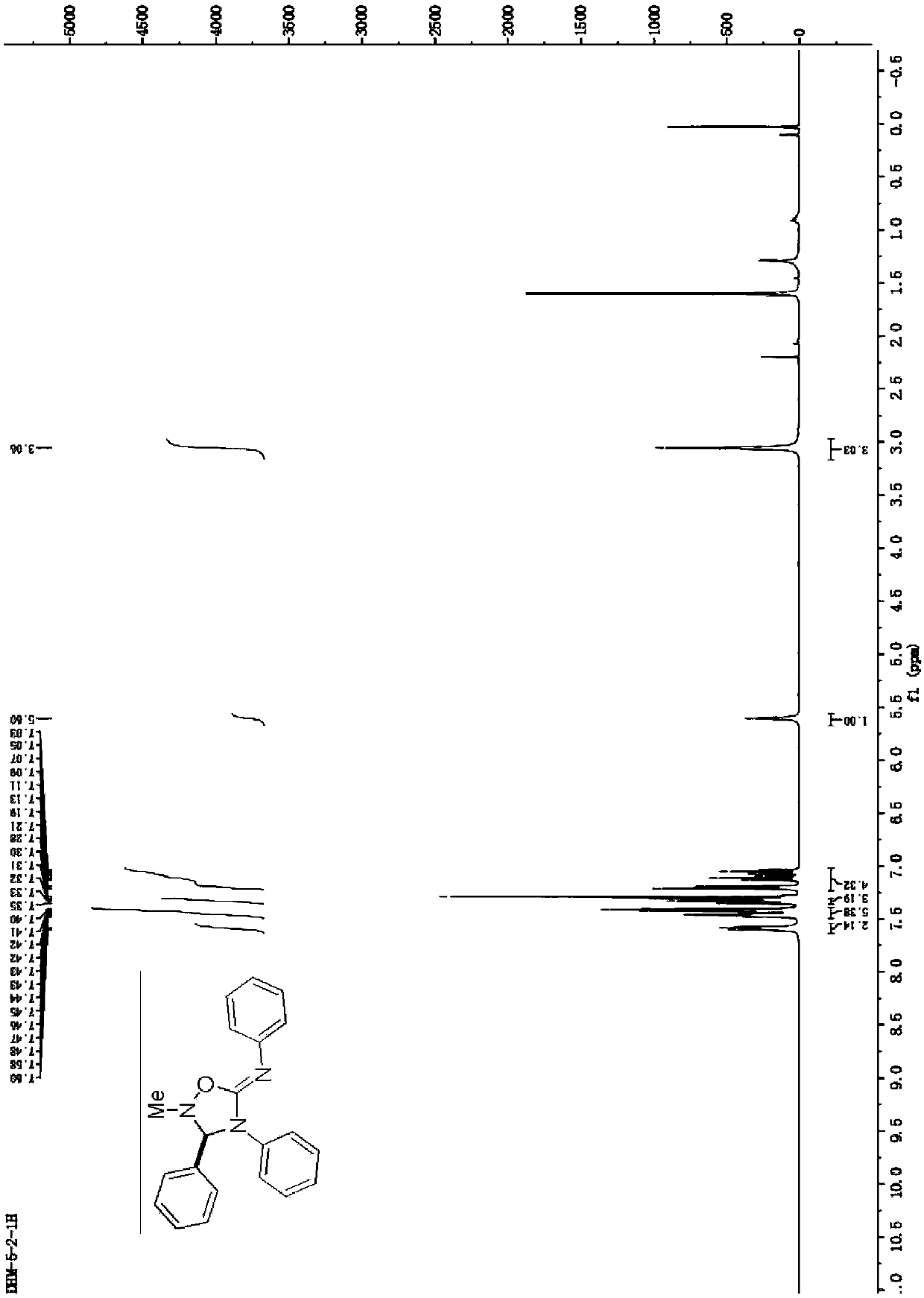
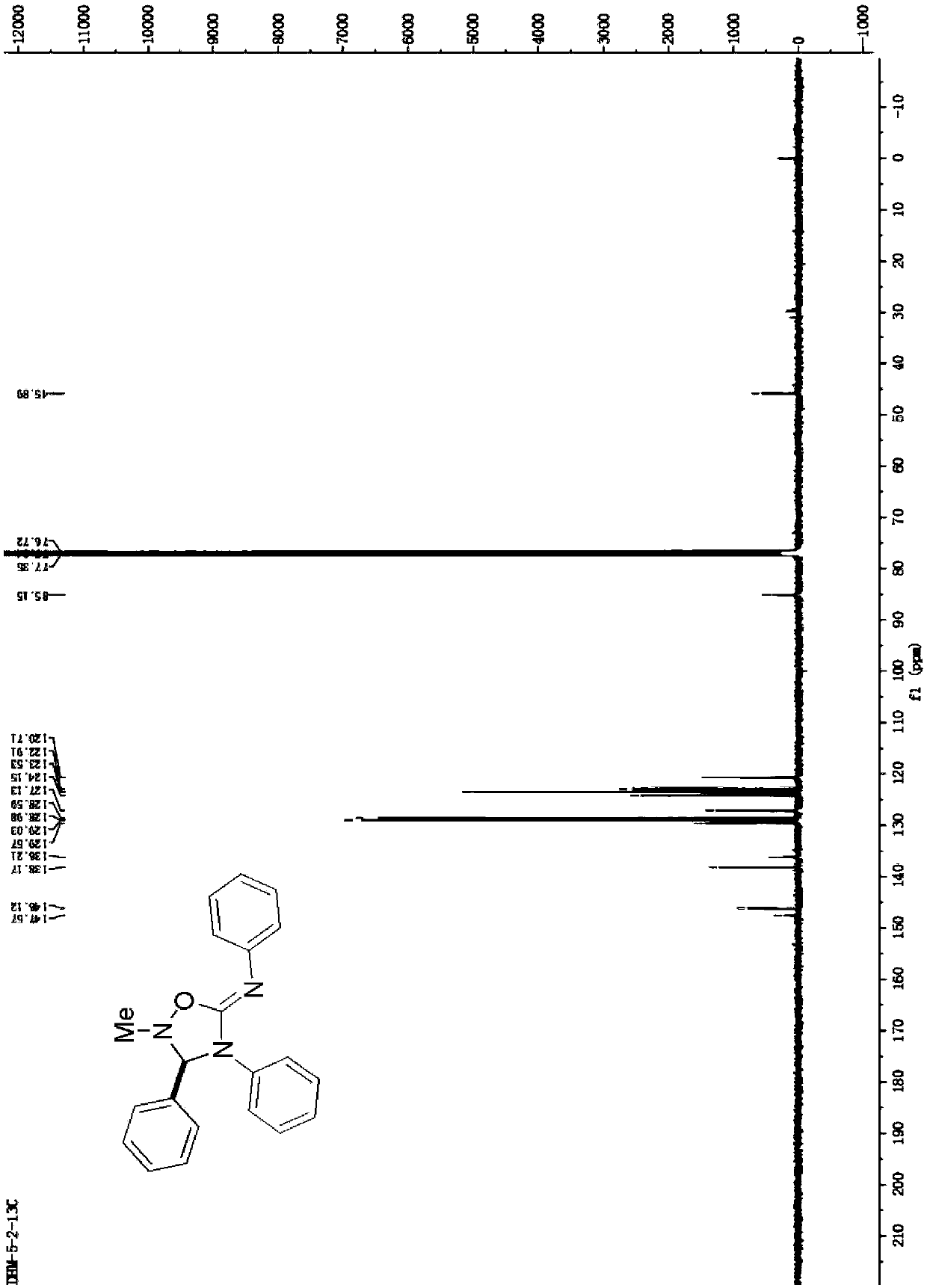
[0028] Synthesis of 2-methyl-N,3,4-triphenyl-1,2,4-oxadiazole-5-imine
[0029] Add 1.5mmol of N-methyl-1-phenylcarbodiimide oxide into the reaction vessel, then add 2ml of acetonitrile, 0.10mmol of diphenylcarbodiimide compounds, and react at 60°C. After the reaction, wash with aqueous solution After washing, extraction with an organic solvent, drying, and distillation under reduced pressure to remove the solvent, the crude product was separated by column chromatography to obtain the target product with a yield of 92%.
Embodiment 2
[0031] Synthesis of 2-methyl-N,4-diphenyl-3-(o-tolyl)-1,2,4-oxadiazole-5-imine
[0032] In the reaction vessel, add 1.5mmol of N-methyl-1-o-tolyl imine oxide, then add 2ml of acetonitrile, 0.10mmol of diphenylcarbodiimide compounds, and react at 60°C. After the reaction, use Washed with aqueous solution, then extracted with organic solvent, dried, concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain the target product with a yield of 86%.
Embodiment 3
[0034] Synthesis of 2-methyl-N,4-diphenyl-3-(p-tolyl)-1,2,4-oxadiazole-5-imine
[0035] In the reaction vessel, add 1.5mmol of N-methyl-1-p-tolyl imine oxide, then add 2ml of acetonitrile, 0.10mmol of diphenylcarbodiimide compounds, and react at 60°C. After the reaction, use Wash with aqueous solution, then extract with organic solvent, dry, evaporate and concentrate under reduced pressure to remove the solvent, and the crude product is separated by column chromatography to obtain the target product with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com