Preparation method of pharmaceutical composition for inhalation and product thereof
A composition and drug technology, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as difficult process implementation, increased production costs, and unfavorable drug manufacturers, and achieve Good particle stability, avoiding solvent residue problems, and good powder fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0123] In the preparation method of the present invention, the equipment used in the solid mixing step can be either a low-shear mixer or a high-shear mixer.
[0124] In one embodiment of the invention, the low shear mixer may be mixer.
[0125] In another embodiment of the invention, the high shear mixer may be mixer.
[0126] In yet another embodiment of the invention, the high shear mixer may be mixer.
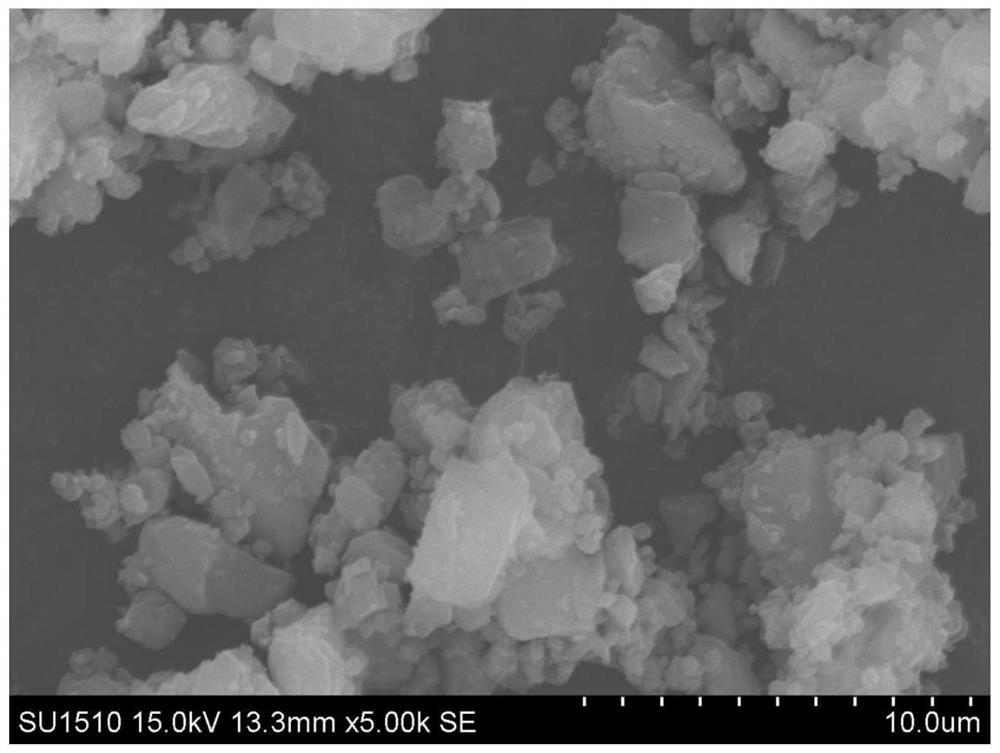
[0127] In some cases, the preparation method of the present invention may further include a jet pulverization step, which is used to pulverize the mixture of the pharmaceutically active ingredient and part of the pharmaceutically acceptable carrier (such as a glidant) to obtain a co-gas powder (or a powder containing medicine powder).
[0128] For example, when the pharmaceutically acceptable carrier is a combination of sugar and sugar alcohols (such as lactose monohydrate) and lipids (such as magnesium stearate), the jet milling step can make the pharmaceutical act...
Embodiment 1-16
[0154] Example 1-16: Preparation of remdesivir dry powder inhaler.
[0155] First, weigh each material according to the prescription amount in Table 3; secondly, add magnesium stearate, calcium stearate or stearic acid to remdesivir and mix, jet mill (for example, the shear flow is nitrogen or Air with RH% below 20%, center ring pressure 0.5-12bar, eg 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 or 12.0bar , the Venturi pressure is 1-14bar, such as 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 12.5, 13, 13.5 or 14bar, and the feed rate is 0.1-3g / min, such as 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.3, 1.5, 2.0, 2.5 or 3.0g / min), obtain drug-containing micropowders of different particle sizes (as The magnesium stearate and other components of the glidant have little effect on the particle size measurement of remdesivir, so the particle size measurement results of the drug-containing micropowder can still reflect the particle size of the ...
Embodiment 17-20
[0163] Example 17-20: Preparation of remdesivir dry powder inhaler.
[0164] First, weigh each material according to the prescription amount in Table 5; second, refer to the process conditions in Examples 1-16, add magnesium stearate to remdesivir and mix, and after jet milling, obtain different particle sizes Drug-containing micropowder; finally, the pre-screened lactose and drug-containing micropowder are placed in a solid mixing device for physical mixing to obtain the pharmaceutical compositions for inhalation of Examples 17 to 20.
[0165] Table 4 lists the D of the drug-containing micropowder of each embodiment through particle size analysis-laser diffraction method measurement. 10 、D 50 and D 90 Particle size, the prescription composition of the pharmaceutical composition of each embodiment is listed in table 5.
[0166] Table 4. Particle size distribution of the medicinal ingredients of Examples 17 to 20
[0167]
[0168] Table 5. The prescription composition of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com