RAAV vector for expressing antibody IgG1 and application of rAAV vector
A technology of IgG1-raav and vector, which is applied in the field of rAAV vector, can solve the problem that the cutting efficiency of the linking polypeptide is not very high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1. Construction of antibody IgG1-rAAV vector plasmid and verification of its function
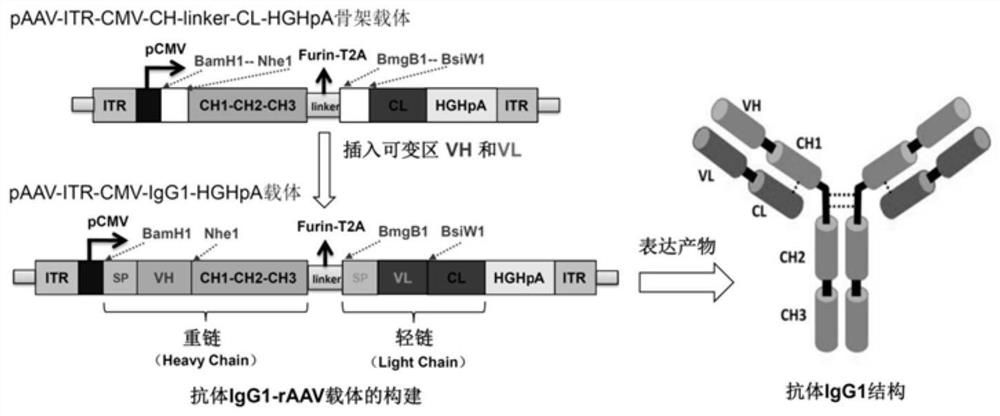
[0017] We chose the strategy of utilizing a single rAAV vector to express IgG1 antibody for optimal design. An antibody expression cassette was constructed on the basis of the pAAV-ITR-MCS plasmid backbone. Wherein, the rAAV terminal repeat sequence (ITR) adopts the ITR sequence of type 2 AAV, as shown in SEQ ID NO.1. Between the two ITR sequences is the expression box for antibody IgG1. We chose the human cytomegalovirus (CMV) promoter with a broad-spectrum strong promoter, followed by the IgG1 antibody heavy chain (Heavy Chain, HC) coding region, and then connected to the IgG1 antibody light chain (Light Chain) through a linker sequence (linker) Chain, LC) coding region, and finally the human serum albumin polyadenylate (HGHpA) sequence.
[0018] Since the differences between different IgG1 antibodies are mainly in the heavy chain HC variable region VH and heavy chain LC...
Embodiment 2
[0022] Example 2. Packaging and purifying antibody IgG1-rAAV virus
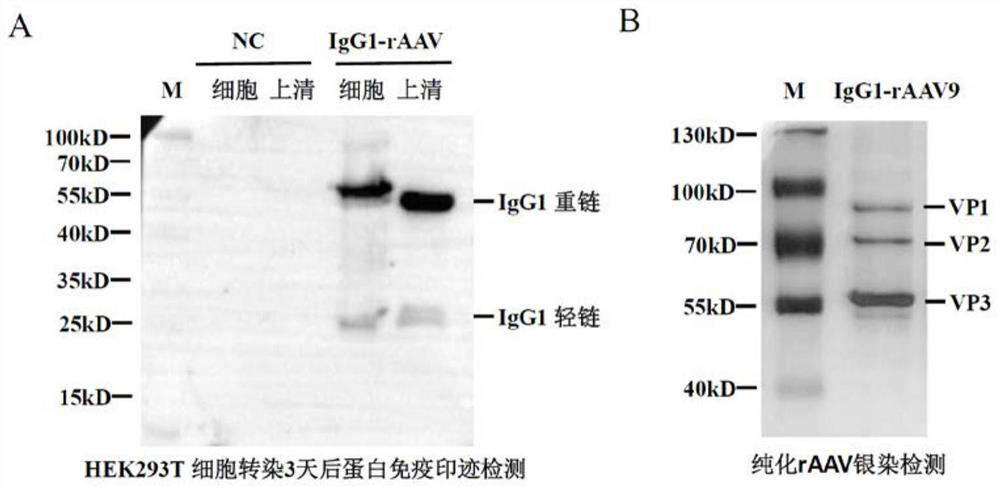
[0023] We prepared antibody IgG1-rAAV by transfecting HEK 293T cells with three plasmids. In order to facilitate packaging and in vivo testing, we selected the serotype plasmid pAAV-RC9 of type 9 AAV for experiments. First, spread HEK293T cells on a 10 cm culture dish, and when the cells grow to a confluence of about 80%, the pAAV-ITR-CMV-IgG1-HGHpA core plasmid, pAAV-RC9 plasmid and pAAV-Ad-helper plasmid are separated by PEI transfection reagent HEK293T cells were transfected at an equimolar ratio. Three days after transfection, the cells and culture supernatant were collected, in which the packaged IgG1-rAAV9 was present. Then, we used iodixanol (idox) density gradient ultracentrifugation to purify rAAV (refer to Grieger et al., Nat Protoc. 2006; 1(3):1412-28.). The titer of purified IgG1-rAAV9 was detected by fluorescent quantitative PCR to be about 6.5E+12VG / ml. Further, using the method of silver st...
Embodiment 3
[0024] Example 3. Detection of expression of antibody IgG1-rAAV infection in HEK293T cells and C57 mice
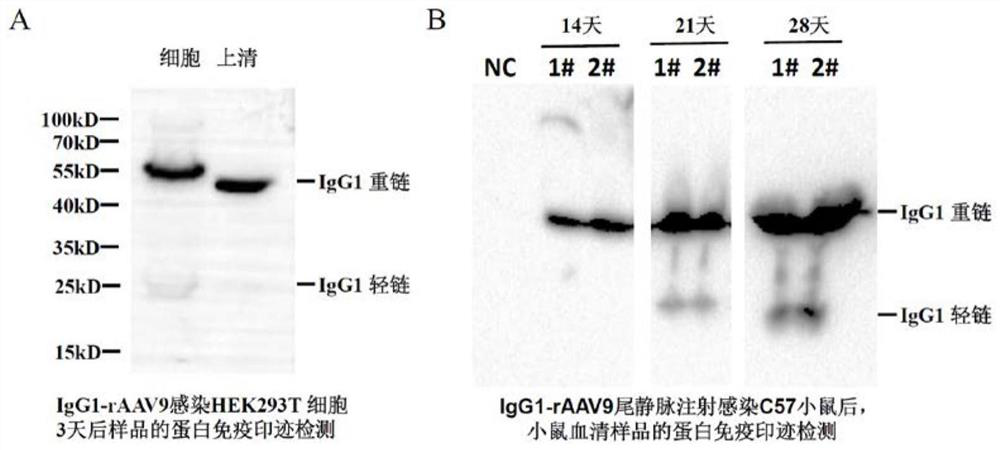
[0025] We used the antibody IgG1-rAAV9 virus obtained in Example 2 to infect HEK293T cells cultured in vitro. Three days after infection, cells and culture supernatants were collected. Then, the cells and supernatant samples were processed and subjected to SDS-PAGE electrophoresis and Western blot detection, and HRP-labeled goat anti-human IgG antibody was used to recognize the expressed product IgG1. The experimental results showed that there were obvious specific detection signals of antibody IgG1 heavy chain and light chain in HEK293T cells and supernatant (such as image 3 A).
[0026] We used the antibody IgG1-rAAV9 virus obtained in Example 2 to infect C57 mice by tail vein injection. Blood was taken from the tail vein at 14 days (2 weeks), 21 days (3 weeks) and 28 days (4 weeks) after infection. We placed the blood samples at 37°C for 1 hour, then at 4°C for 12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com