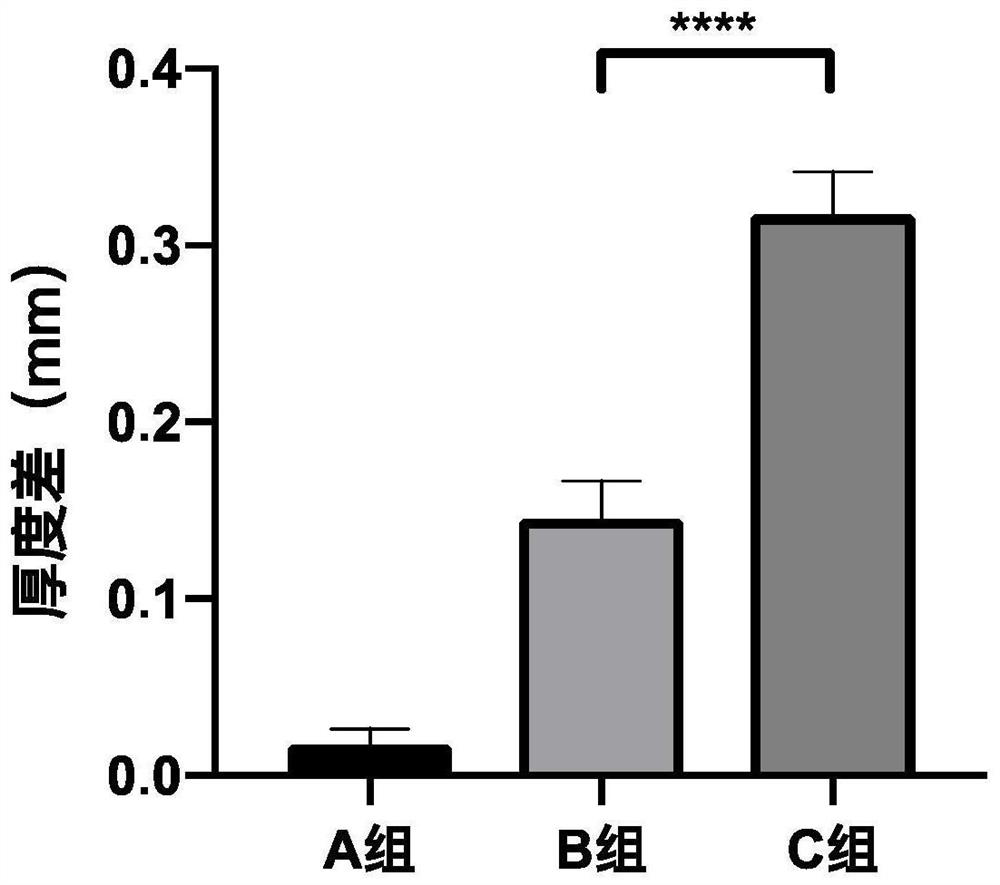
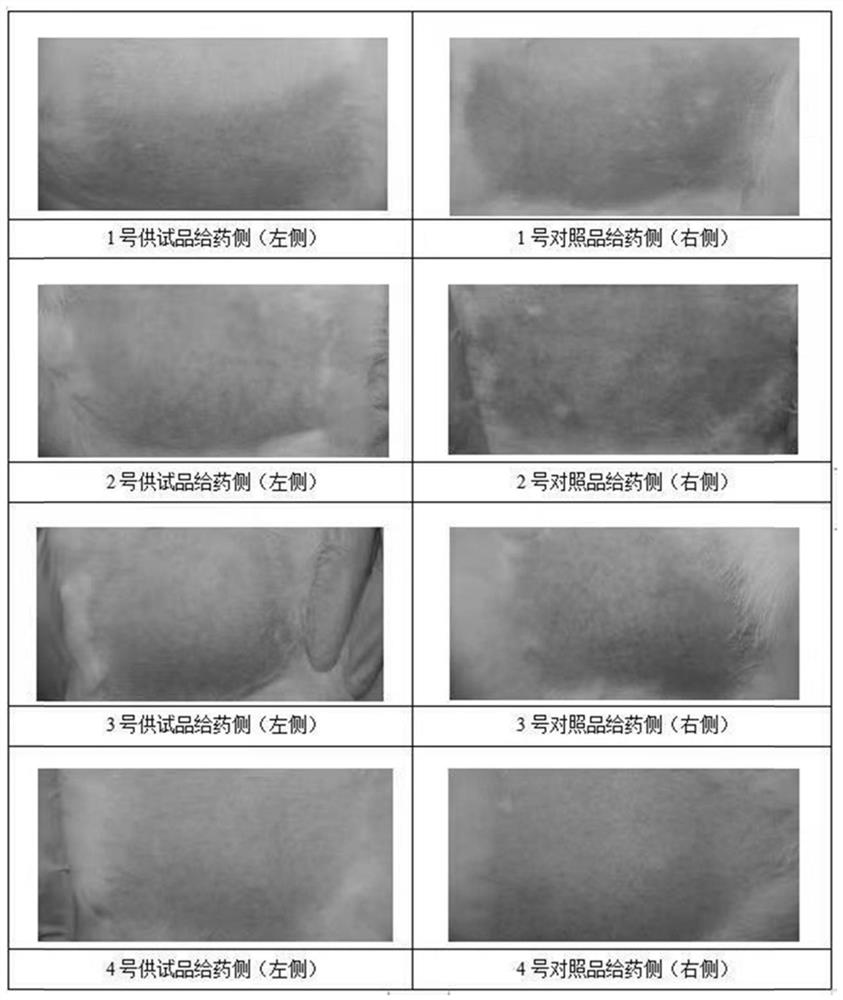
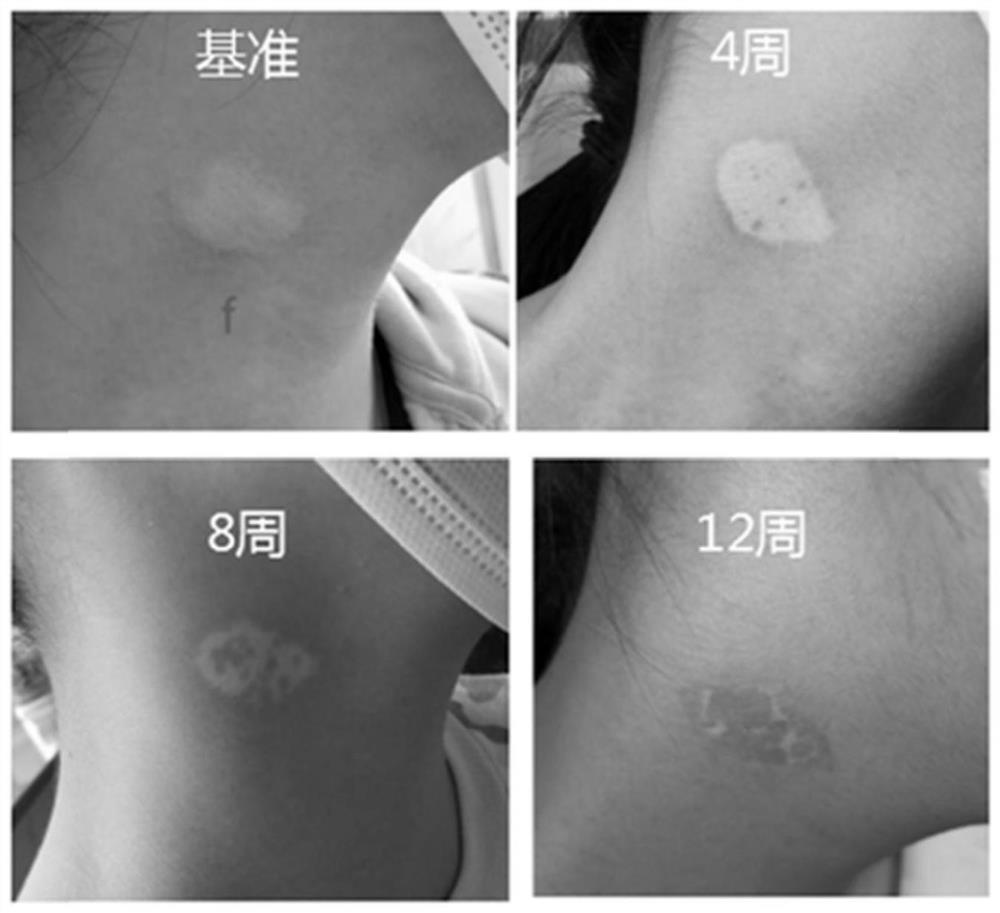
Pharmaceutical composition containing pharmaceutically acceptable salt of tofacitinib, preparation and application
A technology of tinib pharmacy and tofacitinib, applied in the field of pharmaceutical compositions of salts, can solve problems such as poor stability of free state tofacitinib, and achieve the effects of good drug efficacy, optimal drug exposure, and simplified processing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Synthesis of Torker
[0098] Synthesis of tartaric tartaribini (1)
[0099] A 25 ml flask was added 500 mg (1.6 mmol) free trustteibi and 288 mg (1.9 mmol) tarttic acid, 7.5 ml of acetone, stirred and heated to 40 ° C for 2 h, cooled to room temperature, filtered and collected, and normal temperature was dried. The product was 717 mg, yield 96.9%.
[0100] Lagory synthesis of tartaric acid Trust Dibini (2)
[0101] Weighing free trust replacement 45.0g in a single-mouth reaction bottle, 25 ° C ± 5 ° C, add 21.6 g of L-tartaric acid and 225 ml of methanol, heating to reflux, after the solid is dissolved, heat filtration, recover the mother liquor, concentrate to the dry Then, then 450 ml of n-hexane was stirred at 25 ° C ± 5 ° C, stirring, and filtered to obtain a solid, 70 ± 5 ° C for 48 h in a blower oven to obtain a tartaric acid trunk, yield of 95.0%.
[0102] Synthesis of sulfuric acid tunibi
[0103] A 25 ml flask was added 500 mg (1.6 mmol) free state trunk...
Embodiment 2
[0106] Example 2: Preparation of different external formulations
[0107] Table 1-1 Tarten Tourist Digent Cream Formula
[0108]
[0109]
[0110] Note: In Table 1-1, the percentage of each component in the aqueous phase and the oil phase and the outer phase is calculated based on the total amount of the oil phase and the aqueous phase.
[0111] Table 1-2 Tourness Tourist Dibini's cream formula
[0112]
[0113] Note: The percentage of each component in the aqueous phase and the oil phase and the outer phase in the B6 to B11 is calculated based on the total amount of the oil phase and the aqueous phase.
[0114] Table 1-3 Tourness Trust Trust Dibini's cream formula
[0115]
[0116] Note: The percentage of each component in Table 1-3 is calculated based on the total amount of the oil phase, the aqueous phase, and the outer phase.
[0117] Table 1-4 Tarten Tourist Dieni's cream formula
[0118]
[0119] Note: The percentage of each component in Table 1-4 is calculated bas...
Embodiment 3
[0140] Example 3: Dissolution of Different Salt Types Dissolved Properties Test
[0141] In order to improve the transdermal ability of the partial preparation and the stability of the drug, the solubility of the drug is very critical in the excipient, so the solubility of the raw material is an important indicator of the drug. Therefore, in the development of prescriptions, a balanced solubility study is carried out: taking excess raw materials (citrate Trust Dibini, tartaric acid Trust Dibin, sulfate Thaifini, phosphate Tutibi) placed in 2 ml PE In the addition, different solvents were added, dissolved at room temperature, and the solubility was calculated using high performance liquid chromatography after 24 h.
[0142] Table 4: Solubility of different salt types in different solvents
[0143]
[0144]
[0145] In the table: ">" means "greater than", "<" means "less than".
[0146] This trial, we accidentally found that in a monoethyl ether of the permeable dietary dihydrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com