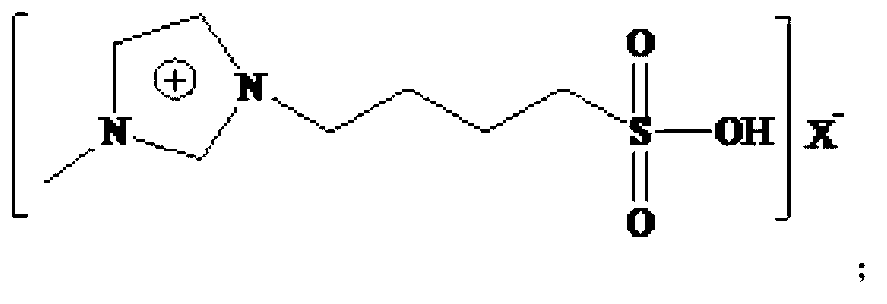
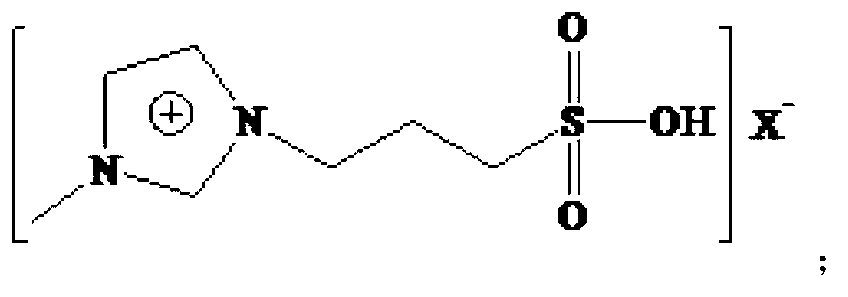
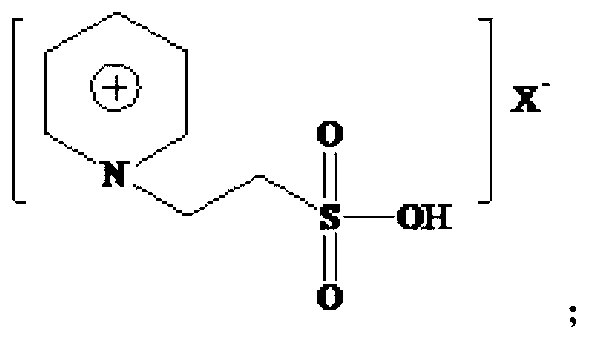
Technical method for preparing 2-ethyl-2-hexenal by catalyzing self-condensation of n-butyraldehyde with acidic ionic liquid
The technology of an acidic ionic liquid and a process method, applied in the field of green chemistry, can solve the problems that the liquid alkali catalyst cannot be reused, the stability of the solid alkali catalyst is poor, and the cost of treating the three wastes is high, achieves good industrial application prospects, and overcomes the cumbersome post-processing process. , the effect of high thermal stability and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] other The preparation method of acidic ionic liquid is the same.
[0042] The Lewis acidic ionic liquid involved in the present invention is characterized by chloroaluminate acidic ionic liquid, prepared by the method described by Wikes et al. (Inorg.Chem., 1982, 21:1263). With [Bmim]Cl-AlCl 3 For example:
[0043] (1) Add the purified N-methylimidazole and chlorobutane into a three-necked flask at a molar ratio of 1:1-1:1.5, and react for 24-48 hours at a reaction temperature of 60-80°C , washed and dried to obtain light yellow viscous [Bmim]Cl.
[0044] (2) Under nitrogen protection, [Bmim]Cl was added to the three-necked upper bottle, and AlCl 3 The molar ratio of [Bmim]Cl to [Bmim]Cl is 1:1-3:1 and slowly add anhydrous AlCl in batches 3 , stir evenly, and react at 60-80°C until brown transparent [Bmim]Cl-AlCl is obtained 3 .
[0045] The preparation method of other Lewis acidic ionic liquids is the same.
Embodiment 1
[0047] In a 100ml autoclave, put 40g of n-butyraldehyde, and then add [Bmim]Cl-ZnCl with 1% n-butyraldehyde weight 2 ionic liquid, with N 2 Air replacement, magnetic stirring, and reaction at 150°C for 5 hours. After the reaction, the reaction solution was left to stand. After the reaction solution was separated, the upper layer liquid was taken for gas chromatography analysis. The conversion rate of n-butyraldehyde was 72.7%. The selectivity of -2-hexenal is 59.5%.
[0048] Examples 2-80 were carried out according to the operation steps of Example 1, and the reaction conditions and results are shown in the summary table.
[0049]
[0050]
[0051]
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com