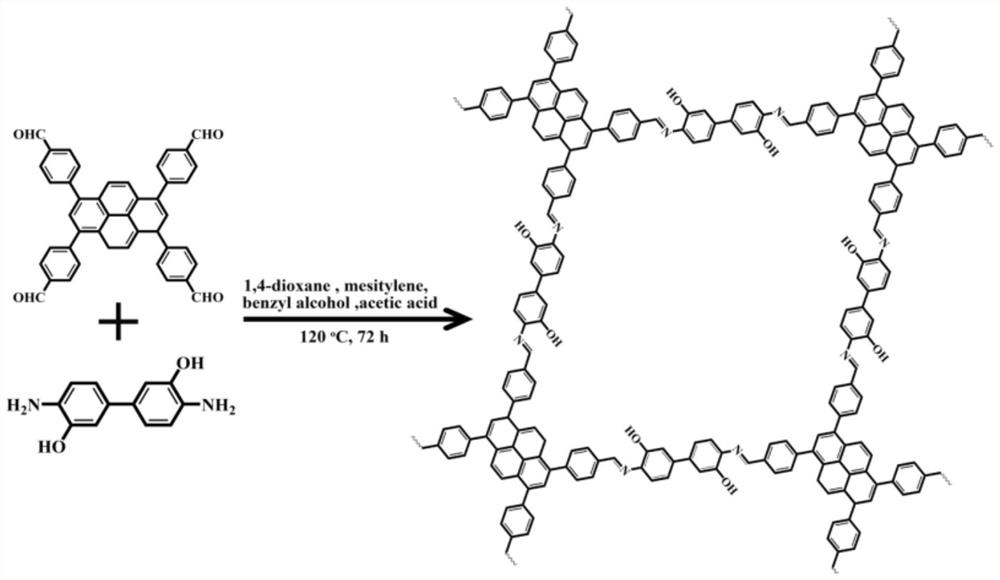
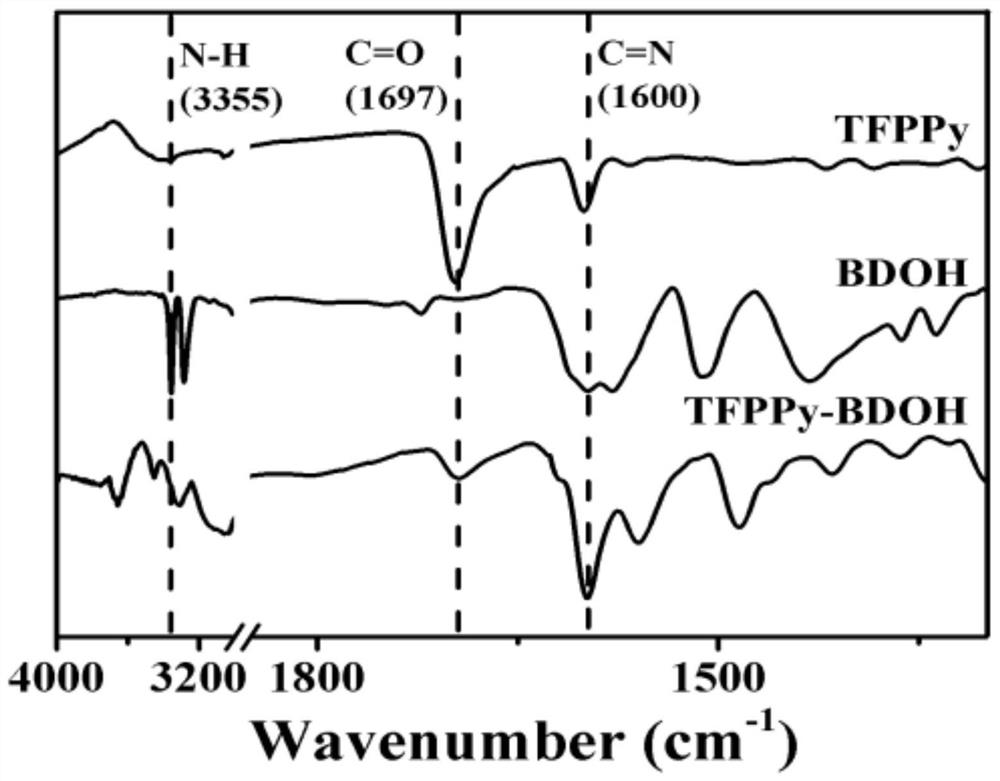
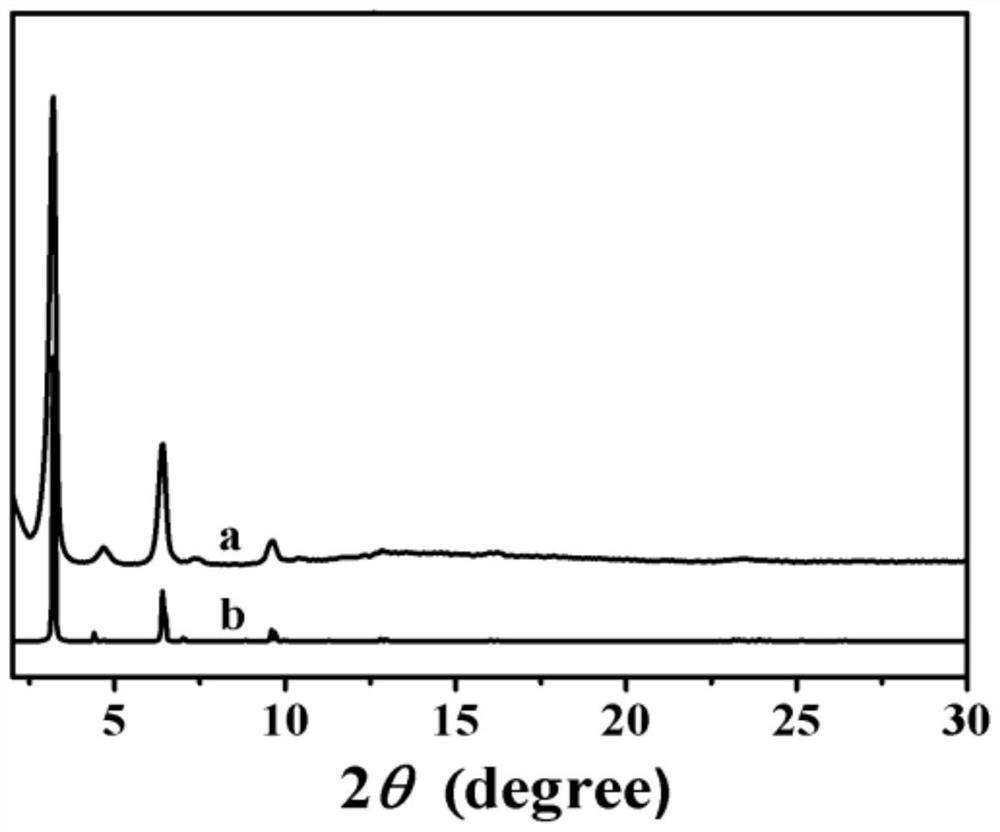
Preparation method and application of imine bond connected fluorescent covalent organic framework
A technology of covalent organic framework and imine bond, which is applied in the field of preparation of fluorescent covalent organic framework, can solve the problems of reducing porosity and crystallinity, and achieve the effects of increasing adsorption capacity, reducing cost and high availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation and Characterization of Fluorescent Covalent Organic Framework Linked by Imine Bonds
[0031] 1,3,6,8-Tetrakis(4-formylphenyl)pyrene (TFPPy) (24.7 mg, 40 μmol) and 4,4'-diamino-[1,1'-biphenyl]-3,3 '-diol (BDOH) (17.3 mg, 80 μmol) was loaded into a 20-mL Pyrex tube, and then 1,4-dioxane (800 μL), benzyl alcohol (800 μL), mesitylene (1334 μL) and acetic acid solution ( 200 μL, 6M); the suspension was sonicated for 10 min, then frozen in a 77K liquid nitrogen bath, and after degassing through three freeze-pump-thaw cycles, the tube was evacuated and flame-sealed; the tube was placed in an oven at 120 °C Heated under the conditions for 72 hours, cooled to room temperature, collected the solid product after filtration, washed several times with tetrahydrofuran (THF), and dried to obtain a solid, which was vacuum-dried at 80°C for 12 hours to prepare a fluorescent covalent organic compound linked by an imine bond. Framework (TFPPy-BDOH).
[0032] fig...
Embodiment 2
[0035] Example 2: Detection of uranyl ions by TFPPy-BDOH
[0036] Make TFPPy-BDOH into 0.025mg mL -1 The N,N-dimethylacetamide (DMAC) dispersion liquid, take 360 μL of the dispersion liquid, add 40 μL of uranyl ions of different concentrations, so that the final concentration of uranyl ions is 0-25 μM, after shaking evenly, use fluorescence spectrophotometry The fluorescent signal of the mixed solution was tested under the excitation wavelength of 325nm by a meter.
[0037] Figure 4 It is the fluorescence response diagram of TFPPy-BDOH to different concentrations of uranyl ions. Depend on Figure 4 It can be seen that with the uranyl ion (UO 2 2+ ) concentration increases, the fluorescence signal of TFPPy-BDOH decreases gradually, 25μM UO 2 2+ The fluorescence quenching rate of TFPPy-BDOH can reach more than 90%. Fluorescent signal of TFPPy-BDOH and UO 2 2+ Concentration is linear in the range of 0-25μM for UO 2 2+ The detection limit of 8.8nM. In addition, the...
Embodiment 3
[0038] Example 3: Adsorption capacity of TFPPy-BDOH to uranium
[0039] Add 5mg of TFPPy-BDOH to a solution containing 10-300ppm uranyl ions, shake in a shaker at a constant temperature for 12h, take 1mL of the suspension, filter it with a 0.22μm microporous membrane, collect the filtrate, and use inductively coupled plasma mass spectrometry Measure remaining UO in filtrate 2 2+ content, the final calculation is TFPPy-BDOH to UO 2 2+ The adsorption capacity of 982.6 mg / g is higher than that of most existing materials. For example, the adsorption capacity of the carboxyl functionalized zinc MOF material developed by Liu et al. is 114.7 mg / g (R. Liu, Z.-Q. Wang, Q.-Y. Liu, F. Luo, Y.-L. Wang.A zinc MOF with carboxylate oxygen-functionalized pore channels for uranium(VI)sorption, Eur.J.Inorg.Chem., 2019, 735-739), Sun et al. have an adsorption capacity of 408 mg / g based on amidoxime-based COF materials (Q.Sun ,B.Aguila,L.D.Earl,C.W.Abney,L.Wojtas,P.K.Thallapally,S.Ma.Covalen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com