Preparation method of hard-core soft-membrane type nano slow-release drug delivery system for injection
A nano-sustained release and drug delivery system technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients. and quality control issues, to achieve the effect of simple structure design and preparation process, high safety, easy sterilization and scale-up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
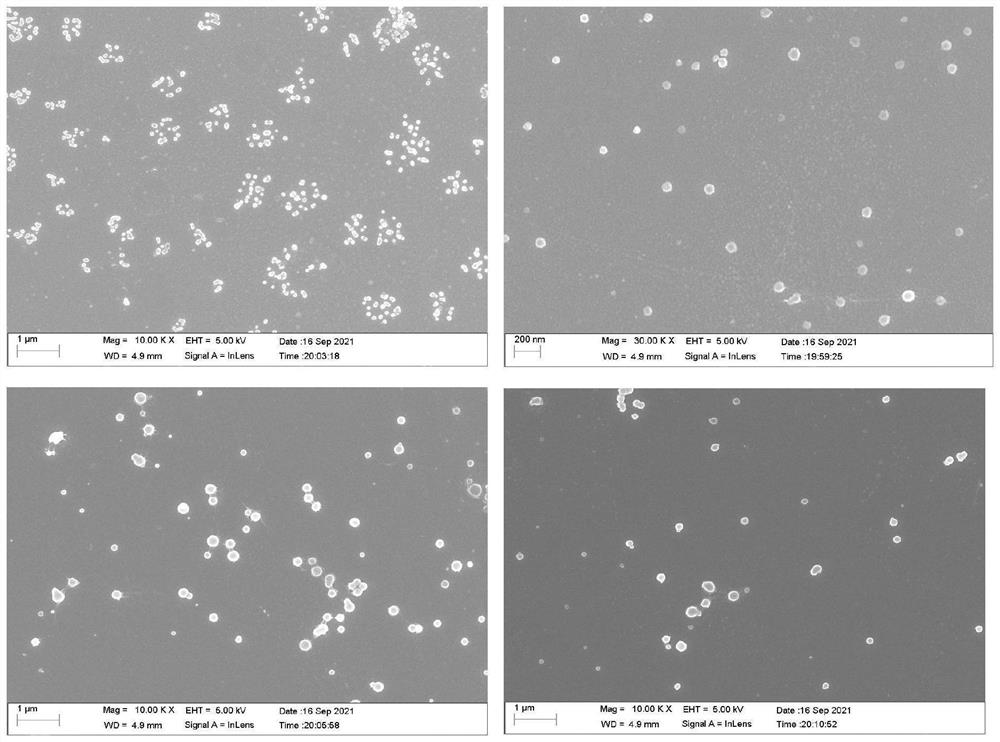
[0031] put H 3 PO 4 The aqueous solution was added to the Ca(OH) under stirring condition 2 Suspension, maintain the pH value of the system in the range of 9-12. The reacted precipitate was frozen at -25°C, placed at 0°C for vacuum drying, and the obtained powder was calcined at 900°C to make HAP powder.
[0032] Mix L-aspartic acid, phosphoric acid and deionized water in a round bottom flask. The mixture was heated to 170° C. under reduced pressure on a rotary evaporator for 1 hour. When the reaction was complete, the temperature was lowered to 100 °C, and an appropriate amount of N,N-dimethylformamide was added to dissolve the residue. It was slowly dropped into 500 mL deionized water at room temperature. The product was collected by filtration, washed with deionized water and dichloromethane.
[0033] Dissolve 1.0g of the prepared PSI in N,N-dimethylformamide to make a PSI solution for later use, take 8.0g of HAP hydroxyapatite and put it in a beaker, add an appropria...
Embodiment 2
[0035] Dissolve 5mL of Tween-80 in 25mL of toluene, add 60mL of 1mol / mL Na 2 CO 3 solution, and use a sonicator to sonicate (sonication conditions are 60° C., 35 kW, 20 minutes). The resulting solution was transferred to a burette and slowly added dropwise to 0.2mol / L of CaCl with constant stirring 2 In solution at room temperature, a precipitate was obtained. The precipitate was filtered, washed several times with deionized water and absolute ethanol, and dried at 80 °C for 12 h to obtain CaCO 3 powder. 1 g of synthesized CaCO 3 The powder was added to deionized water (200 mL) and stirred to form a suspension. 200 mL of Na at a rate of 2 mL / min at 60 °C 2 HPO 4(0.03mol / L) was dropped into the solution. The pH of the above solution was adjusted to 11 using 20% NaOH (g / mL) aqueous solution, and stirring was continued at 60 °C for 1 h. The product was collected by filtration, washed 5 times with deionized water and absolute ethanol, and dried at 80 °C for 24 h. HAP w...
Embodiment 3
[0040] Add 0.3mol / L H3PO4 aqueous solution to 0.5mol / L Ca(OH) under high-speed stirring 2 Add dropwise to the suspension to maintain the pH of the system in the range of 9-12. The reacted precipitate was frozen at -25°C, and then vacuum-dried at 0°C to obtain HAP powder.
[0041] L-Aspartic acid (10.0 g), phosphoric acid (85%, 6 mL) and deionized water (1 mL) were mixed in a 250 mL round bottom flask. The mixture was heated to 180° C. under reduced pressure on a rotary evaporator for 1.5 hours. When the reaction was complete, the temperature was lowered to 100° C., and 60 mL of N,N-dimethylformamide was added to dissolve the residue. It was slowly dropped into 500 mL of mechanically stirred deionized water accompanied by ultrasonic conditions at room temperature (stirring rate of 800 rpm, ultrasonic conditions of 55° C., 1600 W). The product was collected by filtration, washed with deionized water and dichloromethane, and dried.
[0042] Weigh 25 mg of PSI and completely d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com