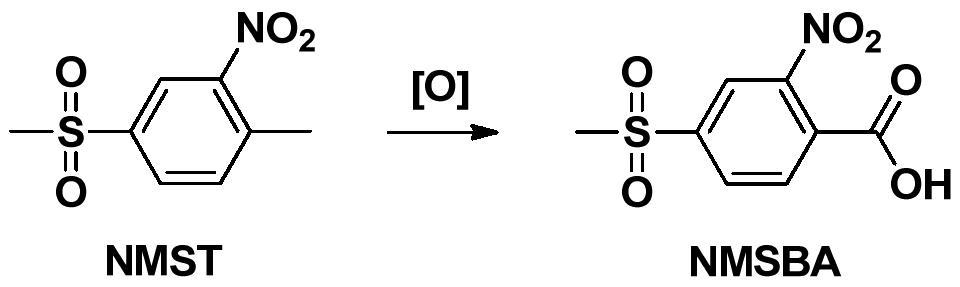
Preparation method of 2-nitro-4-methylsulfonylbenzoic acid
A technology of thiamphenicol benzoic acid and alkyl thiamphenicol benzoate, applied in the field of preparation of 2-nitro-4-thiamphenicol benzoic acid, can solve the problem of poor oxygen reactivity, harsh reaction conditions, and easy pollution Environmental and other issues, to achieve the effect of simple post-processing steps and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
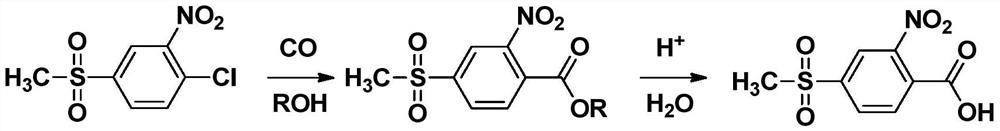
[0025] The invention provides a kind of preparation method of 2-nitro-4-thiamphenicol benzoic acid, the method comprises the following steps,
[0026] 1) In the presence of a base, in the presence of a catalyst and a catalyst ligand, 2-nitro-4-thiamphenicol chlorobenzene is coupled with carbon monoxide and an alcohol with 1-3 carbon atoms to obtain 2-nitro - Alkyl 4-thiamphenicol benzoate;
[0027] 2) the 2-nitro-4-thiamphenicol alkyl benzoate obtained in step 1) is hydrolyzed,
[0028] Wherein, the catalyst is one or more of palladium acetate, palladium chloride, palladium nitrate and copper acetate, and the catalyst ligand is triphenylphosphine, bisdiphenylphosphineethane, bisdiphenylphosphine One or more of propane, bis(diphenylphosphino)benzene, and bis(diphenylphosphino)yldimethylxanthene.
[0029] In the present invention, in the presence of a base, in the presence of a catalyst and a catalyst ligand, 2-nitro-4-thiamphenicol chlorobenzene is reacted with carbon monoxid...
preparation Embodiment 1
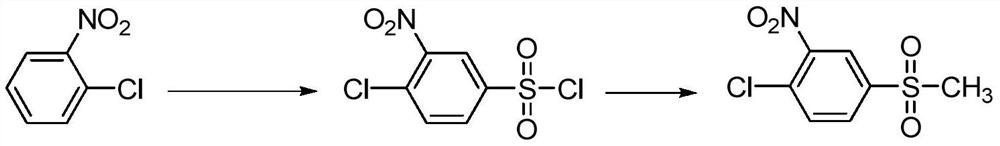
[0063] This example is used to illustrate the preparation of 2-nitro-4-thiamphenicol chlorobenzene
[0064] (1) Add 174.8 grams (1.5 moles) of chlorosulfonic acid, 8.8 grams (0.15 moles) of sodium chloride and 150 ml of 1,2-dichloroethane in sequence in the reaction flask; start stirring, heat, and raise the temperature to 55-60°C ; Then the 1,2-dichloroethane solution (1,2-dichloroethane 250ml) containing 79.8 grams of o-chloronitrobenzene (0.5 moles, 99%) was added dropwise to the above-mentioned mixed solution in 20 minutes medium; after the dropwise addition, keep warm for 5 hours, pour into 500ml ice-water mixture, stir, let stand, and separate the organic layer; dry the organic layer, and precipitate to obtain a light yellow solid 3-nitro-4-chloro Benzenesulfonyl chloride.
[0065] (2) Add 75.6 grams (0.6 moles) of anhydrous sodium sulfite, 400 ml of water and 84 grams (1.0 moles) of anhydrous sodium bicarbonate in sequence in the reaction flask; start stirring, heat to...
Embodiment 1
[0067] This embodiment is used to illustrate the preparation of 2-nitro-4-thiamphenicol benzoic acid
[0068] Put 20.0g of 2-nitro-4-thiamphenicol chlorobenzene, 250ml of methanol and 34.1g of triethylamine into a 1L stainless steel autoclave, add 0.9g of palladium acetate and 2.1g of DPPP, fill with CO gas for replacement three times, and pass into CO to 2MPa, heat up to 170°C, heat and hold pressure for 2 hours, cool down, filter, filtrate is precipitated under negative pressure, add 500ml of water and add 30% by weight hydrochloric acid to adjust the pH to 2, heat up to 50°C, heat for 1 hour, cool down to 20 ℃, filter, wash the filter cake with 25ml of water, dry the filter cake at 90 ℃, get 17.8g product (identified as 2-nitro-4-thiamphenicol benzoic acid through NMR and mass spectrometry), yield 85.2% , a purity of 98.5% by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com