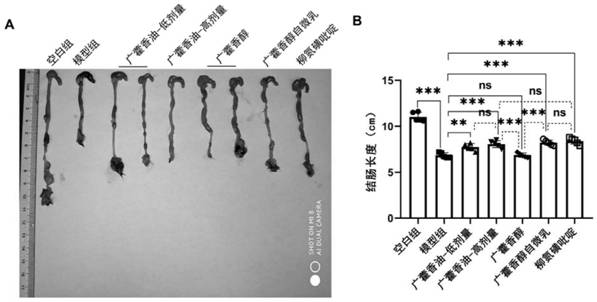
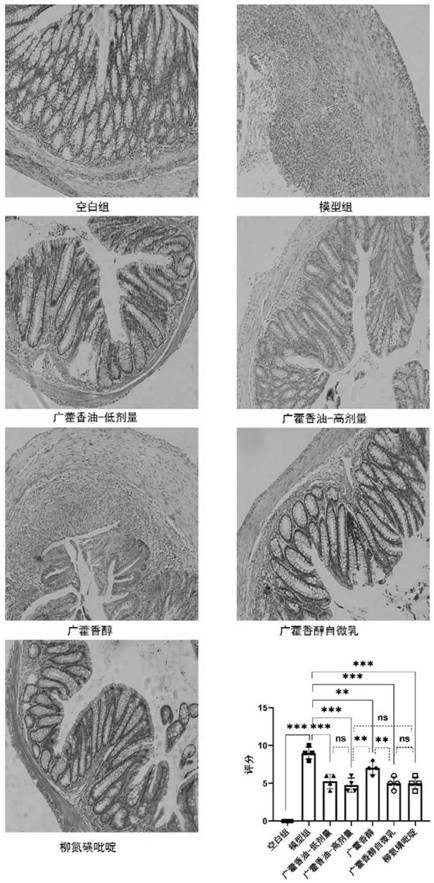
Quality detection method of patchouli oil, clathrate compound of patchouli oil, dry suspension and application of clathrate compound and dry suspension
A quality inspection method, the technology of patchouli oil, is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., to improve drug bioavailability and stability, high in vivo safety, The effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0092] (1) Preparation of reference substance solution: take 1-2 mg of commercially available patchouli alcohol and patchouli ketone reference substances, add n-hexane to dissolve respectively;
[0093] (2) Preparation of the test solution: pipette 50 μL of the commercially available sample, respectively add 200 μL of n-hexane, shake well to obtain the test solution;
[0094] (3) Chromatographic conditions:
[0095] Chromatographic column: Agilent HP-5MS, 30m×0.25mm×0.25μm (serial number USN398724H); temperature program: 70°C at 3°C / min to 150°C, then at 2°C / min to 170°C, then at 5°C ℃ / min increased to 230°C and held for 11min; detector: MS; inlet temperature: 230°C; detector temperature: 250°C; carrier gas: He; column flow rate: 1mL / min; injection volume: 1μL; Ratio: 50:1; Scan type: SCAN; SCAN mode molecular weight range: 50-550.
[0096] (4) Analytical method: use GC-MS technology to measure, analyze the chromatographic and mass spectrum information of the reference subst...
Embodiment 1
[0253] A construction method of patchouli oil GC-MS characteristic spectrum:
[0254] (1) Preparation of reference substance solution: take 1-2 mg of patchouli alcohol and patchouli ketone reference substances, add n-hexane to dissolve them respectively.
[0255] (2) Preparation of the test solution: pipette 50 μL of the commercially available sample, respectively add 200 μL of n-hexane, shake well to obtain the test solution.
[0256] (3) The detection conditions of chromatography and mass spectrometry are shown in Table 9:
[0257] Table 9. GC-MS chromatography and mass spectrometry conditions
[0258]
[0259]
[0260] (4) Analysis and determination: draw 1 μL of the test solution and inject it into the gas chromatography-mass spectrometer for GC-MS detection, analyze the chromatographic and mass spectrometry information of the reference solution and the test solution, and match with the GC-MS NIST database The qualitative attribution information of the 13 character...
Embodiment 2
[0266] Detection of GC-FID fingerprint of patchouli oil:
[0267] (1) Preparation of internal standard solution and reference substance solution: accurately weigh 142.24mg of n-octadecane into a volumetric flask, add n-hexane to make the volume to 10mL, shake well, and set aside; accurately weigh 6.399mg of patchouli alcohol reference substance To the volumetric flask, accurately add 0.2mL internal standard solution, and dilute to 2mL with n-hexane; accurately weigh 4.736mg of patchouli ketone reference substance into the volumetric flask, accurately add 0.2mL internal standard solution, and dilute to volume with n-hexane to 2 mL.
[0268] (2) Preparation of the test solution: pipette 50 μL of a commercially available sample, add 200 μL of n-hexane respectively, and shake well to obtain the test solution.
[0269] (3) Chromatographic conditions are shown in Table 11:
[0270] Table 11. Chromatographic conditions determined by GC-FID method
[0271]
[0272] (4) Analysis ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com