Perch rhabdovirus subunit vaccine and preparation method thereof
A subunit vaccine and rhabdovirus technology, applied in the biological field, can solve the problems of restriction, difficulty in meeting the requirements of large-scale industrialization, enzymatic inactivation, etc., to achieve improved effect, good immunogenicity and immune protection performance, good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Recombinant Escherichia coli E. coli Construction and identification of BL21 / pET32a-G2 strain
[0047] G2 target gene amplification
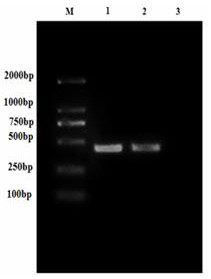
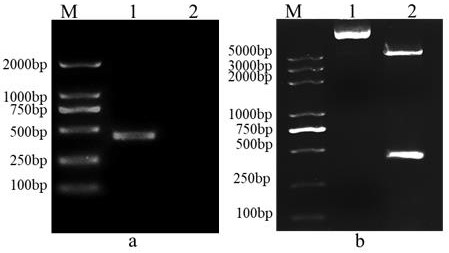
[0048] Referring to the instructions of the viral RNA extraction kit, the MSRV FJ985 strain virus liquid cultured with CO cells (the MSRVFJ985 strain was provided by Northwest A&F University, see Fei Yang et al. Evaluation on the antiviral activity of ribavirin against Micropterus salmoides rhabdovirus (MSRV) in in vitro and in vivo , Aquaculture, Volume 543 , Internet release time: May 27, 2021) as materials to extract MSRV virus RNA. Agarose gel electrophoresis was used to detect the integrity of the RNA, and a micro-nucleic acid analyzer was used to determine the concentration and quality of the sample RNA. The extracted RNA samples were reversed into cDNA using a reverse transcription kit. The G2 gene was amplified with MSRV-G-F / R and VP7-F / R primer pairs, wherein MSRV-G-F: 5'- GAACTGTGGATGGAATAACG-3' (SEQ ID...
Embodiment 2
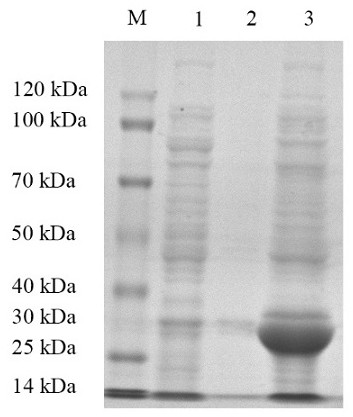
[0065] Example 2: Evaluation of immune efficacy of recombinant protein
[0066] During the research and development process, different fragments were designed, and different glycoprotein fragments and complete glycoproteins were prepared using the method of Example 1, glycoprotein fragment G1 (amino acid sequence is SEQ ID No: 3), glycoprotein fragment G3 (The amino acid sequence is SEQ ID No: 4) protein and the complete glycoprotein G (the amino acid sequence is SEQ ID No: 5), respectively verified the immune efficacy of the above protein fragments. Specifically, the following immune efficacy evaluation test was adopted: 300 healthy perch were randomly divided into 6 groups, including the control group (PBS), the G1 protein group (40 mg / L), the G2 protein group (40 mg / L), and the G3 protein group. (40 mg / L), G protein group (40 mg / L), G protein group (80 mg / L), all sea bass were vaccinated with the corresponding vaccines through the bath solution and soaked for 6 hours. Subs...
Embodiment 3
[0067] Embodiment 3: the preparation of intermediate product for vaccine manufacture
[0068] Production of seed bacterial liquid preparation
[0069] recombinant Escherichia coli E. coli After diluting the basic strain of BL21 / pET32a-G2 strain with normal saline, pick a small amount of bacterial liquid with an inoculation loop, inoculate it in LB liquid medium, culture it at 37°C and 180r / min for 12-16 hours, and harvest the bacteria solution, stored below -20°C.
[0070] Preparation of semi-finished products
[0071] Preparation of first-class seeds: Pick a small amount of bacterial liquid with an inoculation loop for the production strain, inoculate it on the LB solid medium plate by streaking, and culture it at 37°C for 12-16 hours, then pick a single colony and inoculate it on the LB liquid culture culture medium at 37°C and 180r / min for 12-16 hours.
[0072] Preparation of secondary seeds: Inoculate primary seeds in LB liquid medium at 1% (V / V), culture at 37°C and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com