Application of human amniotic epithelial stem cells in preparation of medicine for treating acute kidney injury
A technology for acute kidney injury and human amniotic membrane, applied in the biological field, can solve the problems of short residence time, formation of teratoma, abnormal differentiation, etc., and achieve the effect of reducing the source, easy to obtain materials, and strong proliferation ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Isolation and culture of primary human amniotic epithelial stem cells
[0061] 1 Source of human amniotic membrane
[0062] In order to avoid microbial contamination of the birth canal, fetal placenta was selected by caesarean section. Due to the stimulation of labor signals after term, the amniotic membrane will undergo apoptosis, so it is advisable to use premature fetal placenta (before 38 weeks). After the mother’s authorization and consent, the placental tissue of the healthy mother (HIV, syphilis, hepatitis A, hepatitis B, hepatitis C and other serological reactions were all negative) after caesarean section was taken, the placenta was cut with a cross knife, and the whole amniotic membrane was obtained by mechanical separation.
[0063] 2 Isolation of hAESCs (aseptic operation is required throughout the process)
[0064] The placenta of infants delivered by caesarean section before 39 weeks was obtained, the amniotic membrane was removed from the inne...
Embodiment 2
[0076] Example 2 Establishment of mouse acute kidney injury model
[0077] 1 Preoperative preparation: leave basic hematuria 7 days before operation. Fasting 6h before surgery.
[0078] 2 Bilateral ischemia-reperfusion acute kidney injury (ischemia-reperfusion injury, IRI) model:
[0079] 1) Turn on the temperature control system (36.9-37.1°C); soak surgical instruments in 75% alcohol; prepare surgical accessories, and set a timer.
[0080] 2) The mice were weighed and divided into random groups.
[0081] 3) Anesthesia: 2% sodium pentobarbital, 30-40 mg / kg, intraperitoneal injection.
[0082] 4) Operation: the back skin of the mouse was prepared, disinfected with povidone iodine, fixed on a heating pad in a prone position, and the rectal temperature was measured. Paravertebral longitudinal incision was made, and the skin and muscular layer were cut layer by layer. The kidney was bluntly picked out with a cotton swab, and the renal pedicle was clearly displayed; the renal p...
Embodiment 3
[0086] Embodiment 3 mouse model experiment grouping and processing
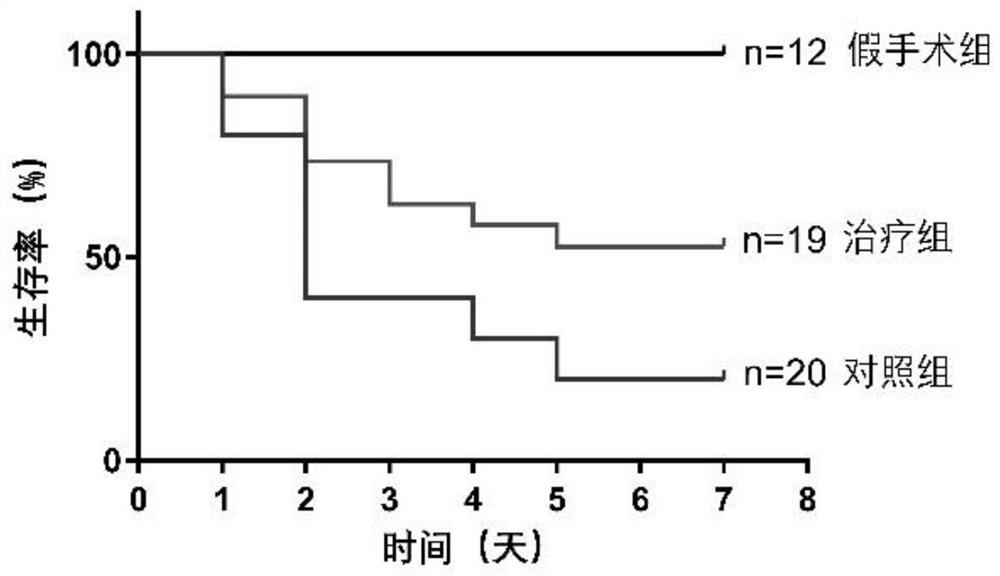
[0087] Group 1: Mice were randomly divided into sham operation group, control group (injection of human amniotic epithelial stem cell preservation solution immediately after operation) and treatment group (injection of human amniotic epithelial stem cell immediately after operation). 18-20 mice in each group.
[0088] 2 Treatment: In the sham operation group, surgical incision was made, the kidney was bluntly picked out with a cotton swab, and then placed back into the abdominal cavity without ischemia-reperfusion operation, and the skin was sutured. Both the control group and the hAECs treatment group completed bilateral renal ischemia-reperfusion surgery. The control group was injected with 100 μl of hAECs cell preservation solution immediately after the operation, and the hAECs treatment group was injected with 100 μl of cell preservation solution resuspended in the tail vein immediately after the operatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com