A kind of preparation method of narcotic analgesic-loaded microspheres, product and use thereof
An analgesic and microsphere technology, which is applied in the field of preparation of narcotic analgesic-loaded microspheres, can solve the problems of low embedding rate, the advent of narcotic analgesic sustained-release microsphere products, and poor drug repeatability, etc. Repeatability, stability, and industrial scale-up effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
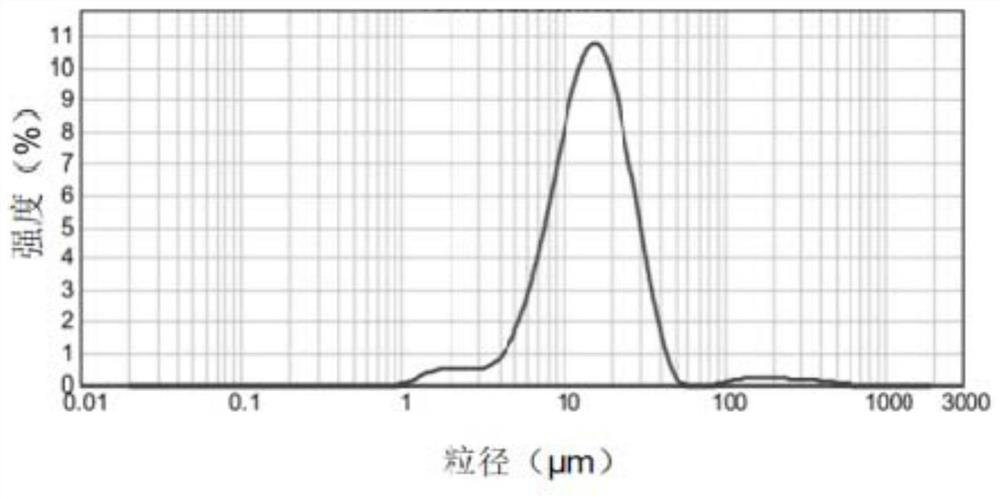
Image
Examples
Embodiment 1
[0101] This embodiment provides a preparation method of anesthesia-analgesic-loaded microspheres, and the preparation method of the anesthetic-analgesic-loaded microspheres includes the following steps:
[0102] (1) A hydrophilic porous membrane with a membrane pore size of 30 μm was soaked in water to fully wet the membrane surface. 75 mg of ropivacaine hydrochloride and 1.5 mg of gum arabic were dissolved in 1 mL of deionized water as the inner aqueous phase (W 1 ), 300 mg of polylactic acid-polyglycolic acid copolymer (PLGA) with a molecular weight of 20,000 (polylactic acid: polyglycolic acid in PLGA = 50:50, molar ratio) and 50 mg of soybean lecithin were dissolved in 10 mL of dichloromethane as oil phase (O). Dissolve 100 mg of polyvinyl alcohol (PVA) in 100 mL of distilled water and stir to be used as the outer water phase (W 2 );
[0103] (2) The inner water phase (W 1 ) was added to the oil phase (O), homogeneously emulsified in an ice bath at 0 °C for 2 min to ob...
Embodiment 2
[0108] This embodiment provides a preparation method of anesthesia-analgesic-loaded microspheres, and the preparation method of the anesthetic-analgesic-loaded microspheres includes the following steps:
[0109] (1) A hydrophilic porous membrane with a membrane pore size of 50 μm was soaked in water to fully wet the membrane surface. 50 mg of bupivacaine hydrochloride and 0.05 mg of casein were dissolved in 1 mL of deionized water as the inner aqueous phase (W 1 ), 800 mg of polylactic acid-polyglycolic acid copolymer (PLGA) with a molecular weight of 10,000 (polylactic acid: polyglycolic acid in PLGA = 50:50, molar ratio) and 30 mg of sucrose fatty acid ester were dissolved in 10 mL of ethyl acetate as the oil phase (O). Dissolve 500 mg of polyvinyl alcohol (PVA) in 50 mL of distilled water and stir to be used as the outer water phase (W 2 );
[0110] (2) The inner water phase (W 1 ) was added to the oil phase (O), homogeneously emulsified in an ice bath at 0 °C for 2 min...
Embodiment 3
[0115] This embodiment provides a preparation method of anesthesia-analgesic-loaded microspheres, and the preparation method of the anesthetic-analgesic-loaded microspheres includes the following steps:
[0116] (1) A hydrophilic porous membrane with a membrane pore size of 20 μm was soaked in water to fully wet the membrane surface. 30 mg of lidocaine hydrochloride and 0.3 mg of mannose were dissolved in 1 mL of deionized water as the inner aqueous phase (W 1), 5000 mg of polylactic acid-polyglycolic acid copolymer (PLGA) with a molecular weight of 30,000 (polylactic acid: polyglycolic acid = 75:25, molar ratio) and 50 mg of egg yolk lecithin were dissolved in 10 mL of dichloromethane as oil Phase (O). Dissolve 500 mg of polyvinyl alcohol (PVA) in 150 mL of distilled water and stir to be used as the outer water phase (W 2 );
[0117] (2) The inner water phase (W 1 ) was added to the oil phase (O), homogeneously emulsified in an ice bath at 5°C for 2 min to obtain W 1 / O ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com