Phenylacetone monooxygenase and application thereof in preparation of prazole drugs
A technology of phenylacetone monooxygenase and acetone monooxygenase, which is applied in the application field of phenylacetone monooxygenase and in the preparation of prazoles, can solve the problems such as the overall efficiency difference of esomeprazole, Achieve the effects of improving substrate binding efficiency, improving activity, and reducing engineering costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
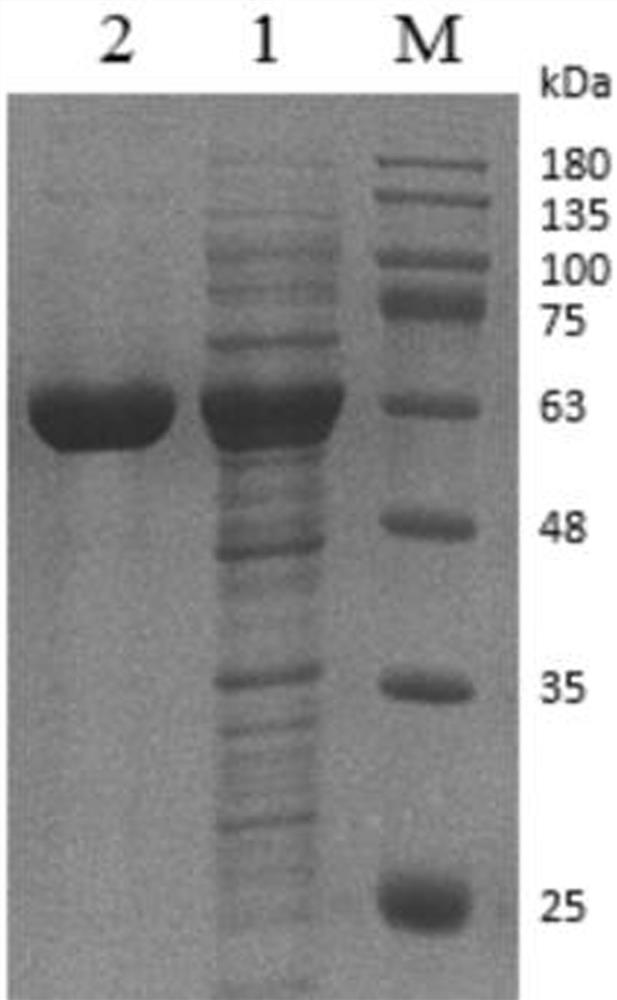
[0049] Example 1 Recombinant expression of phenylacetone monooxygenase
[0050] Screened by gene mining to a source of Limnobacter sp . A novel phenylacetone monooxygenase ( ln PAMO) gene (GenBank No. KYP10950.1), amino acid sequence and reported cyclohexanone propiophenone monooxygenase ( Ac CHMO and CHMO-NCIMB9871) have a low amino acid sequence similarity of 40%. phenylacetone monooxygenase ln PAMO amino acid sequence (SEQ ID NO: 2) was codon optimized to obtain ln The base optimized sequence of PAMO gene is shown as SEQ ID NO:1.
[0051] Synthetic codon-optimized ln The PAMO gene fragment was digested with BamHI and XhoI restriction endonucleases, and connected to the plasmid pET28a to construct the recombinant plasmid pET28a- ln PAMO, and transform the recombinant plasmid into Escherichia coli BL21(DE3) for heterologous expression.
[0052] Heterologous expression: Pick a single clone of the recombinant plasmid on the plate and inoculate it into 25 mL LB li...
Embodiment 2
[0053] Embodiment 2 Propiophenone monooxygenase ln Substrate profiling assays for PAMO whole-cell catalysis
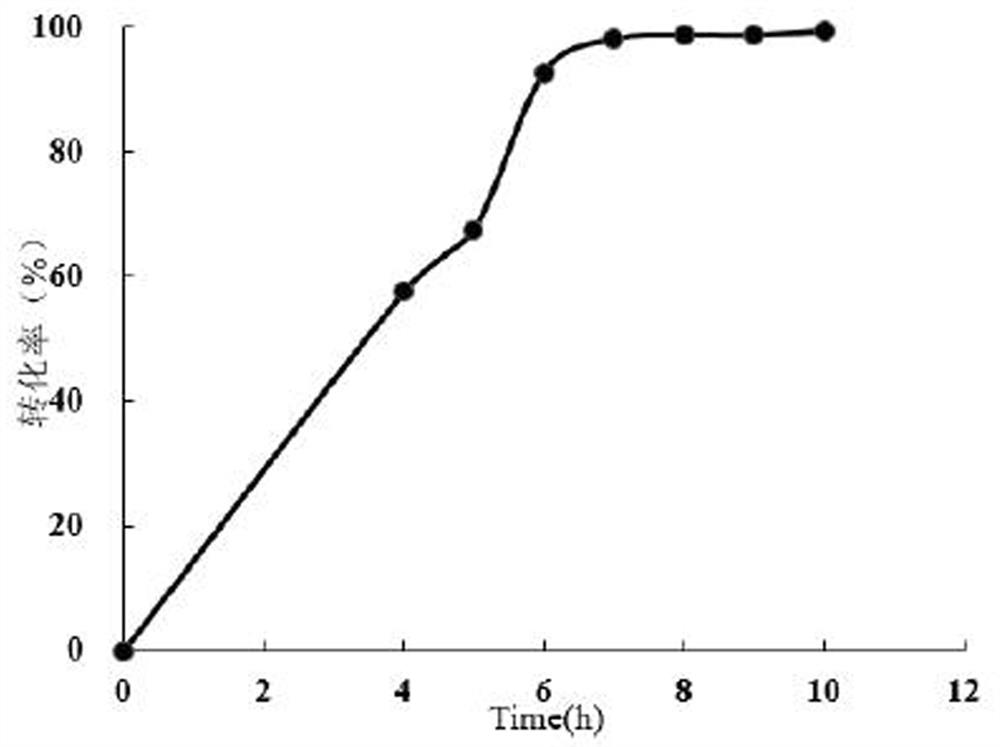
[0054] Take 10 mg of the harvested bacterial cells after induction of expression in Example 1, resuspend with 390 μL of 100 mM Tris buffer at pH 9.0, add 50 μL of 100 mM substrate solution (propiophenone, acetophenone, cyclohexanone, benzyl thioether or omeprazole thioether, final concentration 10 mM, dissolved in methanol), 10 μL 10 mM NADP + (final concentration 0.2 mM), 10 μL glucose dehydrogenase, 10 μL 1 M glucose, placed at 30°C, 1000 rpm for 2 h. The amount of product generated was detected by HPLC, and the conversion rate was calculated. The liquid chromatography conditions for the thioether substrate reaction are: C18 reversed-phase column, mobile phase is acetonitrile: water = 53:47, flow rate 1 mL / min, column temperature 30°C, detection wavelength 254 nm, detection time 13 min . Gas phase detection conditions: 30 m*0.32 mm*0.5 μm C18 gas phase column, h...
Embodiment 3
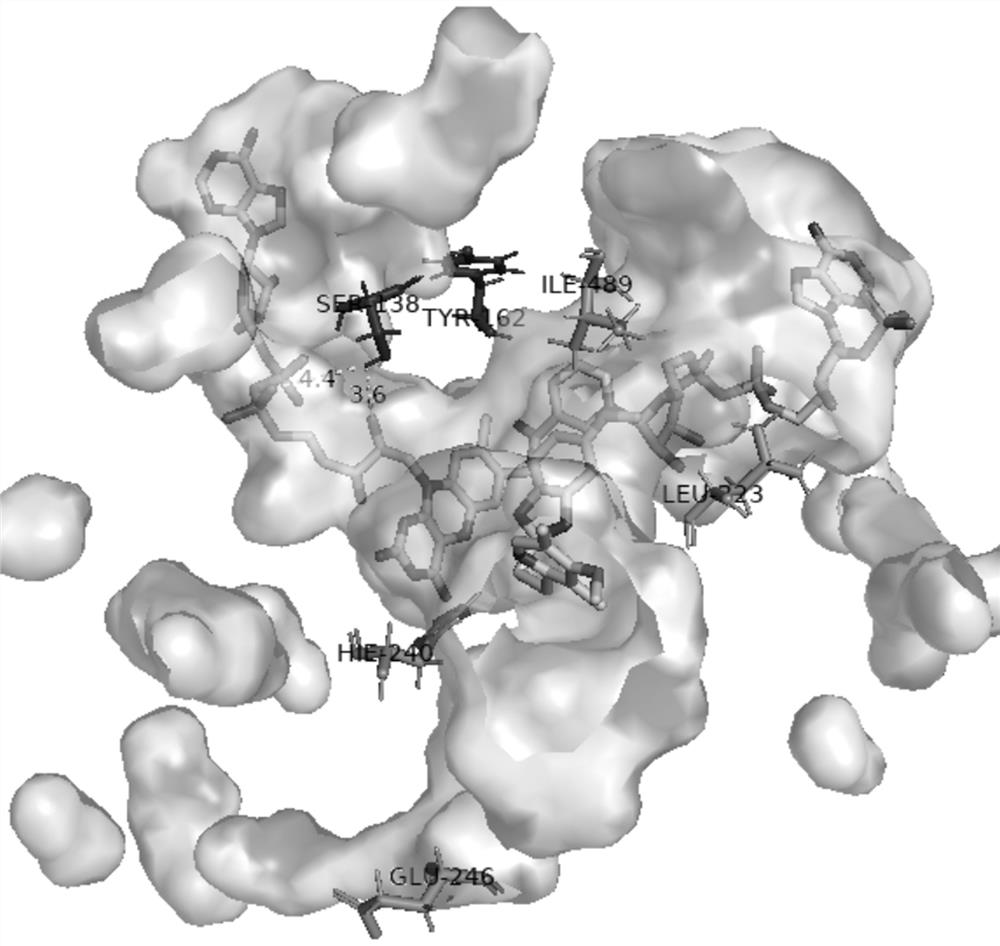
[0058] Embodiment 3 Propiophenone monooxygenase ln Site-directed mutagenesis of PAMO
[0059] Rational design, protein homology modeling based on amino acid sequence (SEQ ID NO: 2), and molecular docking construction ln Structural model of the PAMO-coenzyme-substrate complex, examining phenylacetone monooxygenase separately ln Amino acid residues within 5 angstroms of the FAD and NADP ligand-binding regions of PAMO ( figure 2 ). Analyze each secondary force, and through site-directed mutagenesis, mutate amino acid residues at targeted positions to enhance ln Binding effect of PAMO on the coenzymes FAD and NADP. In addition, in the substrate channel region, the key amino acid residues that recognize and bind to the substrate omeprazole thioether are analyzed, and the amino acid residues are mutated at key positions through site-directed mutagenesis to promote the effective recognition of omeprazole thioether by the substrate channel , to increase the substrate binding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com