Method for synthesizing glycolide
A technology of glycolide and methyl glycolate, which is applied in the field of polymer monomer preparation, can solve problems such as coking, complex catalyst preparation process, and low yield of glycolide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
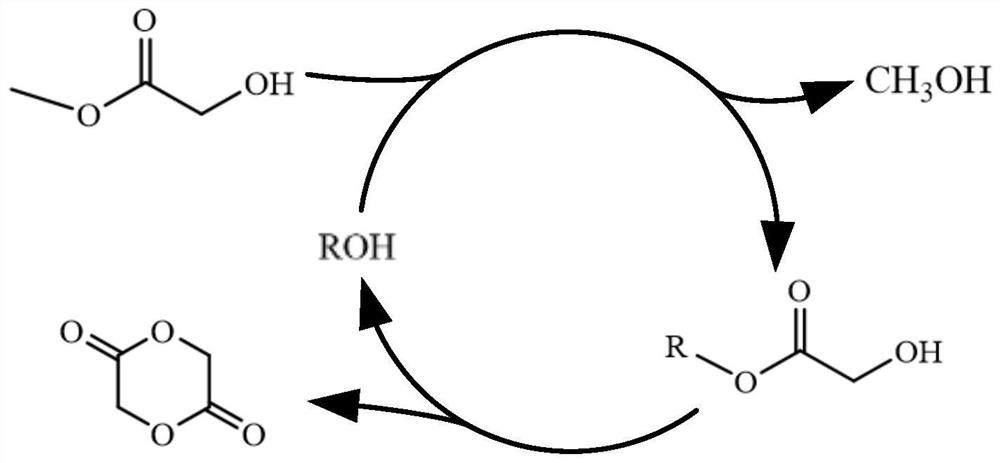
[0042] The synthetic method of the glycolide of the present embodiment comprises the following two steps:
[0043] Step 1: Weigh 16.30g of n-docosanol into a 100mL flask, melt at 60°C, add 4.50g of methyl glycolate and 0.018g of stannous octoate, stir magnetically, and slowly raise the temperature to 140°C until no distillation The effluent was evaporated, and the light yellow liquid in the flask was behenyl glycolate.
[0044] Step 2: Heat the esterification product obtained in Step 1 to 225°C, and vacuumize the system to reduce the pressure to 0.5kPa. At this time, a colorless distillate can be collected, which will condense into white crystals when it is cooled. Wait until there is no liquid distillate out, the reaction was terminated. The distillate is glycolide, weighing 2.07g, and the yield of crude glycolide is calculated to be 71.40%.
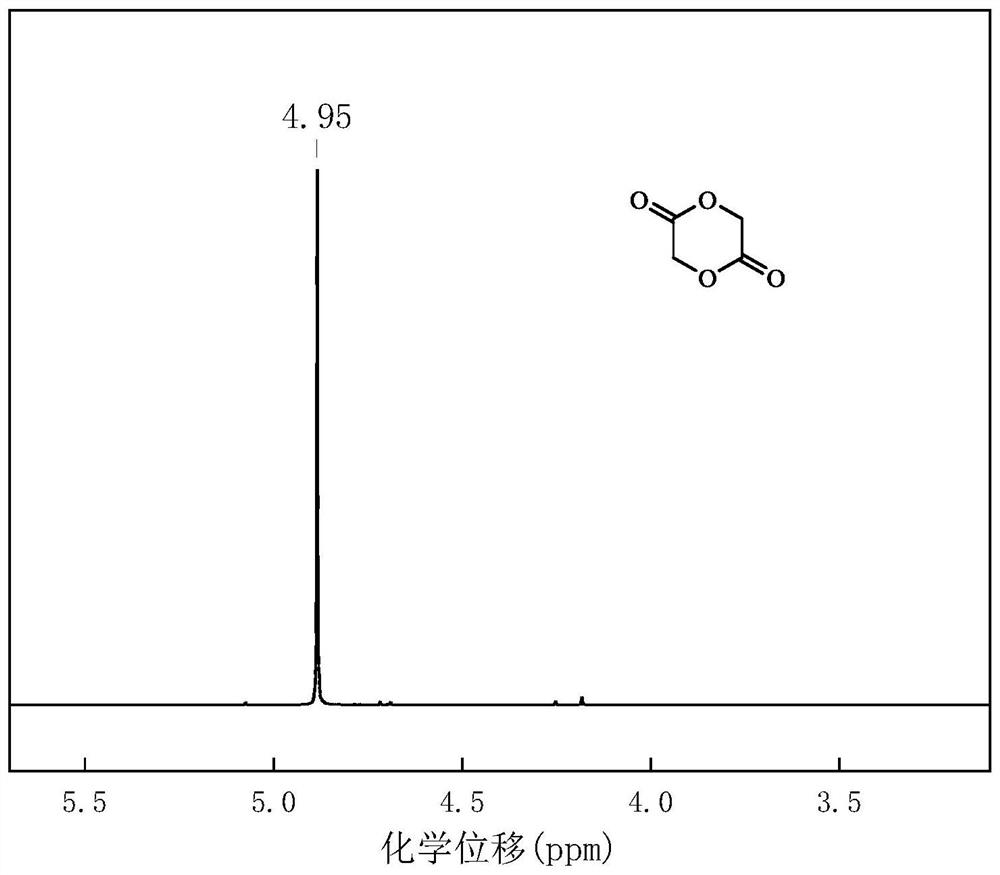
[0045] figure 2 It is the proton nuclear magnetic spectrum of the glycolide prepared by the present embodiment.
[0046] From f...
Embodiment 2
[0061] The synthetic method of the glycolide of the present embodiment comprises the following two steps:
[0062] Step 1: Weigh 14.90g of eicosanol into a 100mL flask, melt at 80°C, add 4.50g of methyl glycolate and 0.018g of stannous octoate, stir magnetically, and slowly heat up to 140°C until there is no distillate After steaming, the light yellow liquid in the flask is eicosyl glycolate.
[0063] Step 2: Heat the esterification product obtained in Step 1 to 210°C, and vacuumize the system to reduce the pressure to 1kPa. At this time, a colorless distillate can be collected, which will condense into white crystals when it is cooled, and wait for no liquid to distill out. , the reaction was terminated. The distillate is glycolide, weighing 1.92g, and the yield of crude glycolide is 66.20%.
Embodiment 3
[0065] The synthetic method of the glycolide of the present embodiment comprises the following two steps:
[0066] Step 1: Weigh 16.30g of n-docosanol into a 100mL flask, melt at 80°C, add 4.50g of methyl glycolate and 0.0675g of stannous chloride, stir magnetically, and slowly raise the temperature to 160°C until no The distillate was evaporated, and the light yellow liquid in the flask was behenyl glycolate.
[0067] Step 2: Raise the temperature of the esterified product obtained in Step 1 to 220°C, and reduce the pressure of the system to 1.5kPa by vacuuming. At this time, a colorless distillate can be collected, which will condense into white crystals when it is cooled. Wait until there is no liquid distillate out, the reaction was terminated. The distillate was glycolide, weighing 2.04g, and the crude yield of glycolide was 70.20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com