Preparation method of viral vaccine, and pharmaceutical composition
A virus vaccine and virus technology, applied in the field of virus vaccine preparation method and pharmaceutical composition, can solve safety problems and other problems, achieve the effect of maintaining immunogenicity, avoiding serious side effects, and antagonizing virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the virus vaccine comprises: providing at least one full-length or partial antigenic peptide or protein coding nucleic acid sequence of the virus; dividing the full-length or partial antigenic peptide or protein coding region into one or more 60 - Partially overlapping nucleic acid sequences of 150 nucleotides to encode 20-50 amino acid residues, each of said partially overlapping nucleic acid sequences encoding sequences with furin cleavage sites or non-immunogenic glycine or serine linkers Separation to make 5-15 amino acid residues overlap; and then connect multiple partially overlapping nucleic acids in series to form nucleic acid vaccine fragments.
[0041] In some embodiments of the present invention, the coding region of the full-length or partial antigenic peptide or protein is divided into one or more partially overlapping nucleic acid sequences consisting of 90-120 nucleotides to encode 30-40 amino acid residues base; each of the part...
Embodiment 1
[0085] Below with embodiment 1 DNA vaccine plasmid in vitro transfection 293T cell experiment, embodiment 2 mouse vaccine injection immunization experiment and embodiment 3 rhesus monkey vaccine injection experiment illustrate the beneficial effect of the present invention.
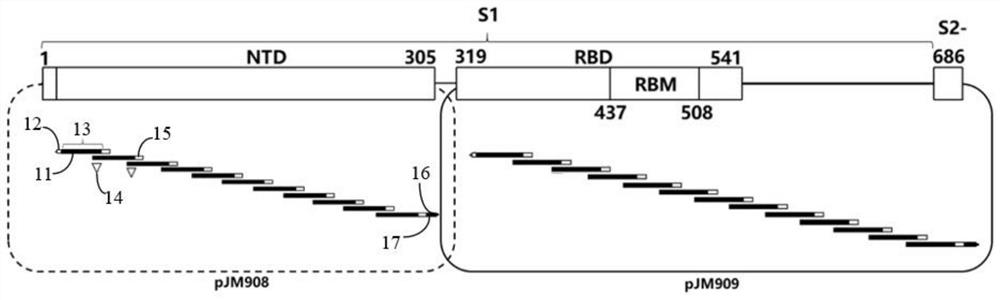
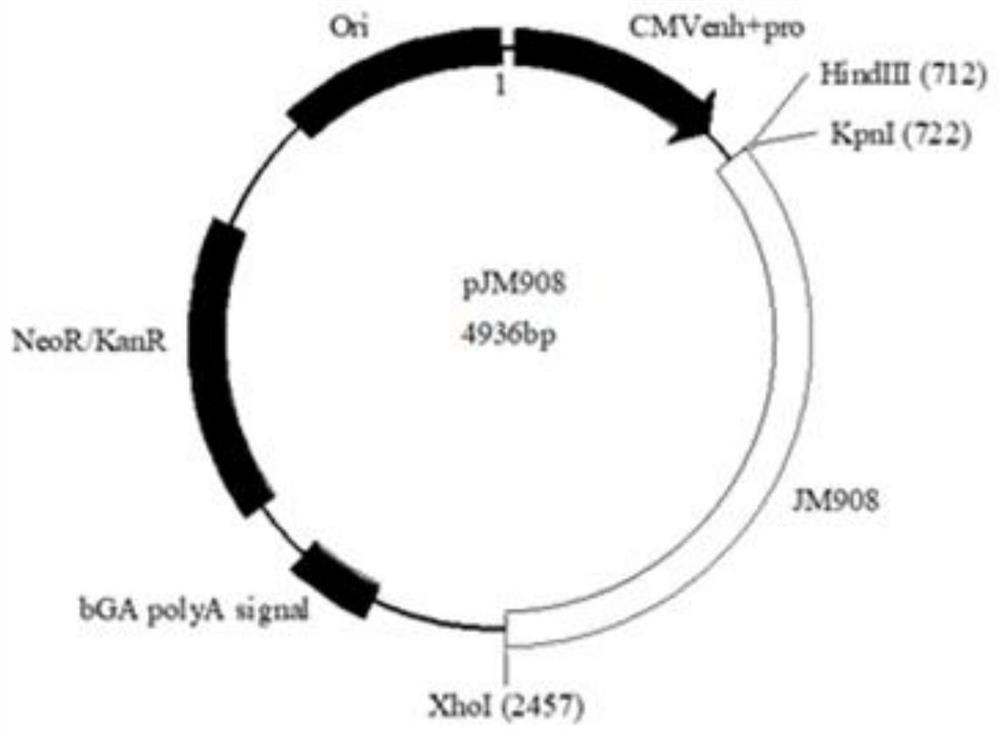
[0086] figure 1 It is a schematic diagram of the partially overlapping nucleic acid sequence structure encoding the novel coronavirus spike protein S1 subunit fragment including the receptor binding domain in the experimental process of some embodiments. Although the S1 subunit has good immunogenicity, it contains more than 650 amino acid residues and may cause serious side effects after entering the human body. refer to figure 1 , pJM908 circular DNA plasmid has S1 subunit NTD encoding nucleic acid fragment, containing 11 partially overlapping nucleic acid sequences, each encoding 36 amino acids, forming a partially overlapping 36 amino acid residue encoding nucleic acid sequence 13, its downstream and ...
Embodiment 3
[0100] Embodiment 3 of the present invention has carried out intramuscular injection to 3-6 years old, the male rhesus macaque of body weight 3.5-8 kilogram with reference to aforementioned mouse vaccine injection immunization experiment, has formed following experimental group:
[0101] Groups 6 and 7: 2 rhesus monkeys were injected with an empty pVax1 plasmid, and the injection volume was 2 mg / mouse;
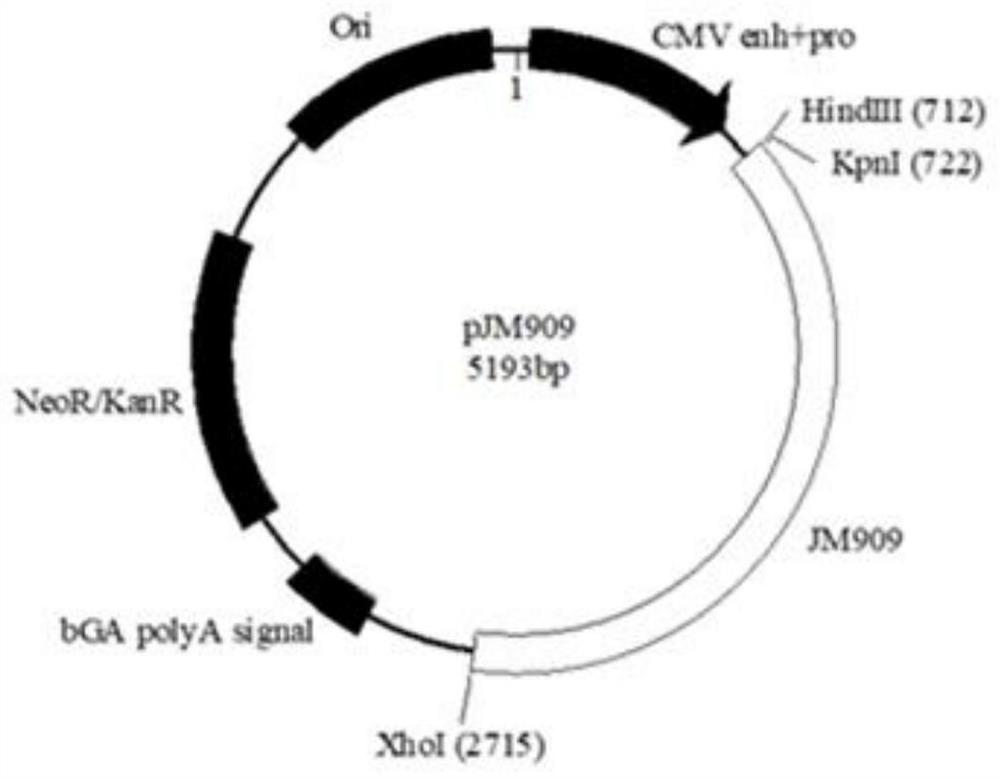
[0102] Groups 8 and 9: 2 rhesus monkeys were injected with pJM909 vaccine separately, and the injection dose was 2 mg / monkey;
[0103] Group 10 and Group 11: 2 rhesus macaques were mixed with pJM908 vaccine and pJM909 vaccine with the same content, and the injection volume was 4 mg / monkey.
[0104] Specifically, immediately after the injection, the muscle cells were electroporated with a Tarisa electroporation device (from Shanghai Tarisa Health Technology Co., Ltd.).
[0105] Specifically, after repeated intramuscular injections of pJM909 and mixed plasmids for 8 weeks in rh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com