Synthetic method of lactam compound
A technology of lactam compound and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of limited expensive catalysts, high temperature, pollution, etc., and achieve the effects of avoiding the use of oxidants or noble metal catalysts, mild reaction conditions, and improving economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
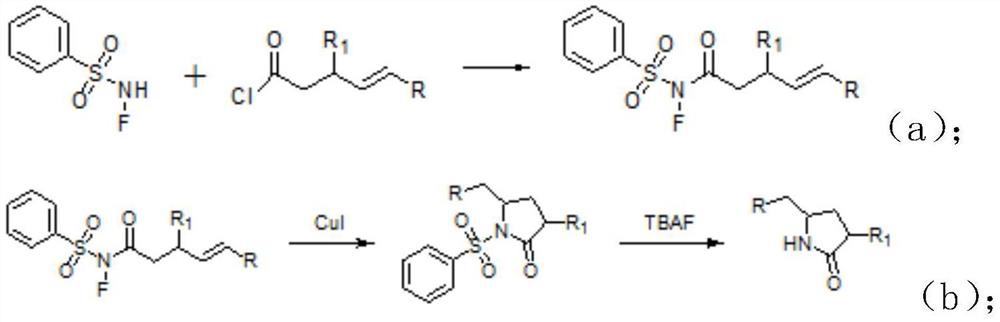
[0029] A kind of synthetic method of lactam compound, comprises the steps: the acid chloride selected in this synthetic method is In the formula, R is an aryl group, R 1 is methyl, that is
[0030] (1) Under nitrogen protection, N-F benzenesulfonamide, (Acyl chloride) was dissolved in dichloromethane and stirred evenly, and stirred and reacted at room temperature for 2.5 hours to obtain (being the crude product of product A); In this reaction step, N-F benzenesulfonamide and The molar ratio is 1:1.2;
[0031] (2) recrystallize the crude product of product A obtained in step (1) to obtain (Product A);
[0032] (3) The product A, cuprous iodide and o-phenanthroline obtained in step (2) are dissolved in a mixed solvent of dichloroethane and methanol and heated at 70°C for 1.5 hours to obtain
[0033] (intermediate); and the molar ratio of the cuprous iodide, the o-phenanthroline and the product A is 1:1.2:10; the volume ratio of dichloroethane and methanol is 10:1;...
Embodiment 2
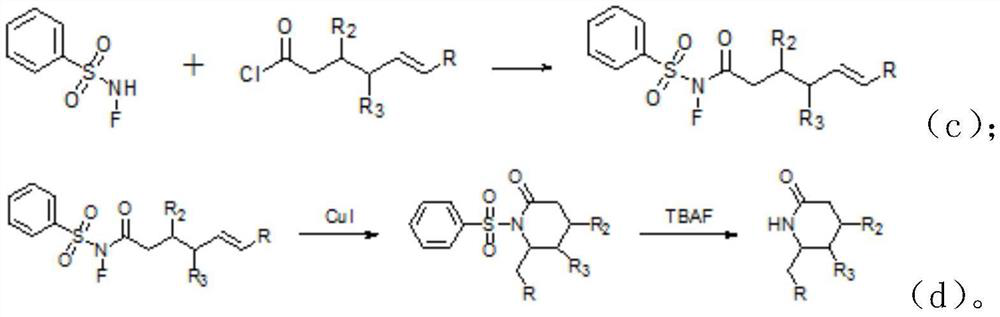
[0038] A kind of synthetic method of lactam compound, comprises the steps: the acid chloride selected in this synthetic method is In the formula, R is an aryl group, R 2 , R 3 is methyl, that is
[0039] (1) Under nitrogen protection, N-F benzenesulfonamide, (Acyl chloride) was dissolved in dichloromethane and stirred evenly, and stirred and reacted at room temperature for 2.5 hours to obtain (i.e. the crude product A); in this reaction step, N-F benzenesulfonamide and The molar ratio is 1:1.2;
[0040] (2) recrystallize the crude product of product A obtained in step (1) to obtain (Product A);
[0041] (3) The product A, cuprous iodide and o-phenanthroline obtained in step (2) are dissolved in a mixed solvent of dichloroethane and methanol and heated at 70°C for 1.5 hours to obtain
[0042] (intermediate); and the molar ratio of the cuprous iodide, the o-phenanthroline and the product A is 1:1.2:10; the volume ratio of dichloroethane and methanol is 10:1;
[...
Embodiment 3
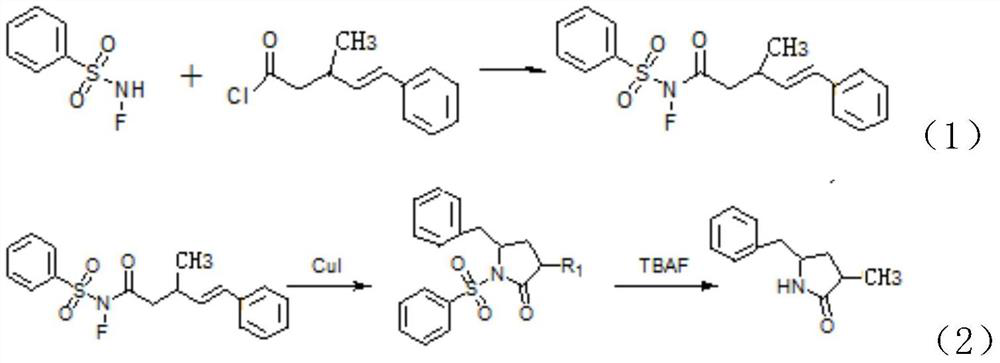
[0047] A kind of synthetic method of lactam compound, comprises the steps: the acid chloride selected in this synthetic method is In the formula, R is an aryl group, R 1 is methyl, that is
[0048] (1) Under nitrogen protection, N-F benzenesulfonamide, (Acyl chloride) was dissolved in dichloromethane and stirred evenly, and stirred and reacted at room temperature for 2.5 hours to obtain (i.e. crude product A); wherein N-F benzenesulfonamide and The molar ratio is 1:1;
[0049] (2) recrystallize the crude product of product A obtained in step (1) to obtain (Product A);
[0050] (3) The product A, cuprous iodide and o-phenanthroline obtained in step (2) are dissolved in a mixed solvent of dichloroethane and methanol and heated at 80°C for 1.5 hours to obtain
[0051] (intermediate); and the molar ratio of the cuprous iodide, the o-phenanthroline and the product A is 0.5:1:10; the volume ratio of dichloroethane and methanol is 10:1;
[0052] (6) Gain from step (3)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com