Fusion protein, vaccine containing fusion protein and application of fusion protein
A fusion protein and vaccine technology, applied in the field of genetic engineering, can solve the problems of weak immunogen, insufficient protection of seasonal influenza vaccine, and high production cost, and achieve the effects of short production cycle, good clinical application value, and wide recognition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
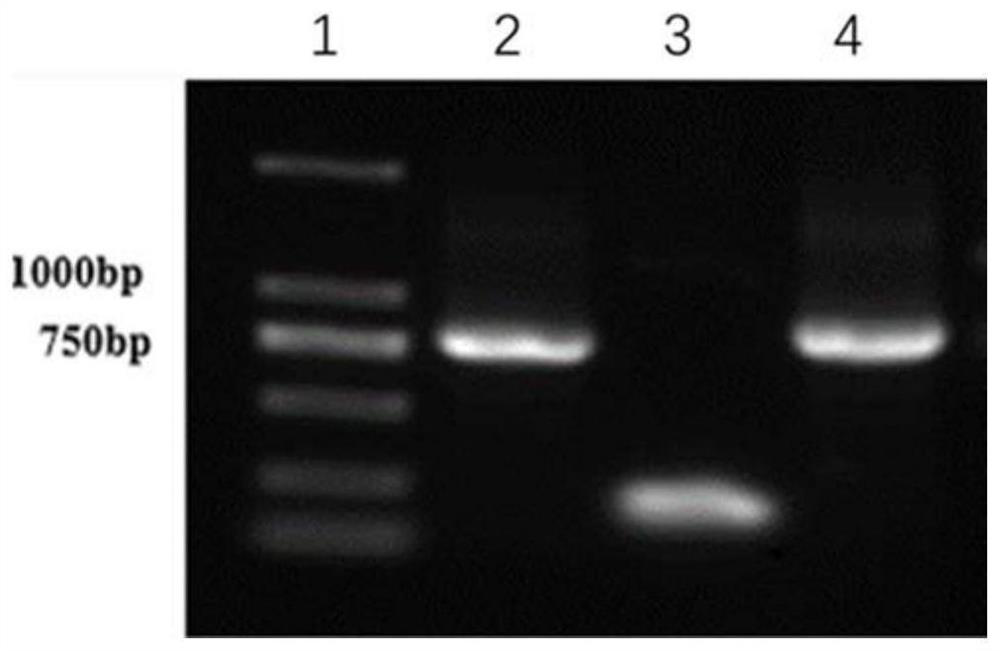
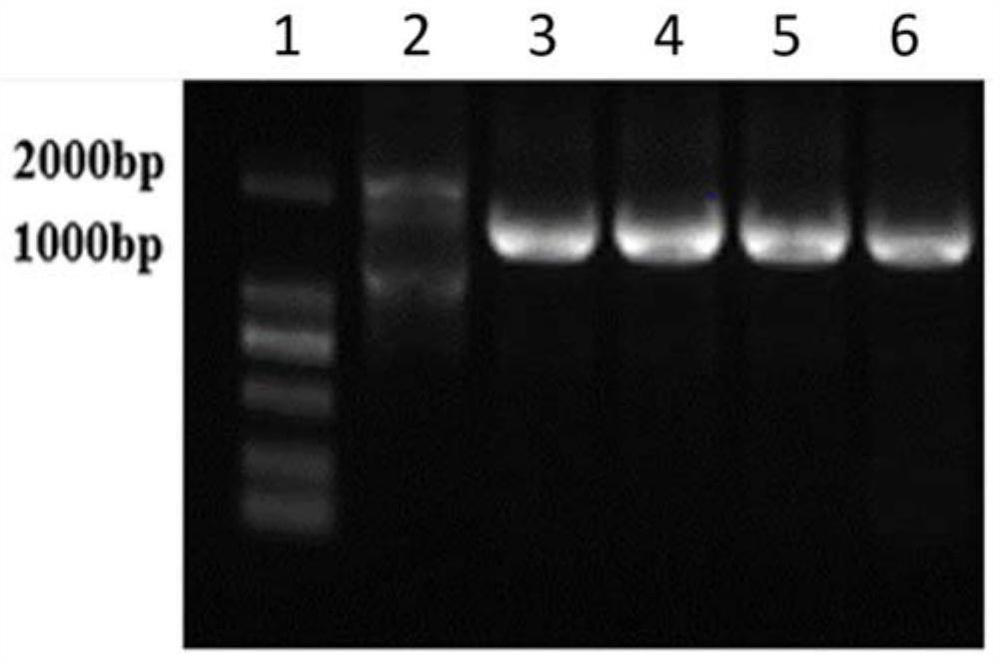
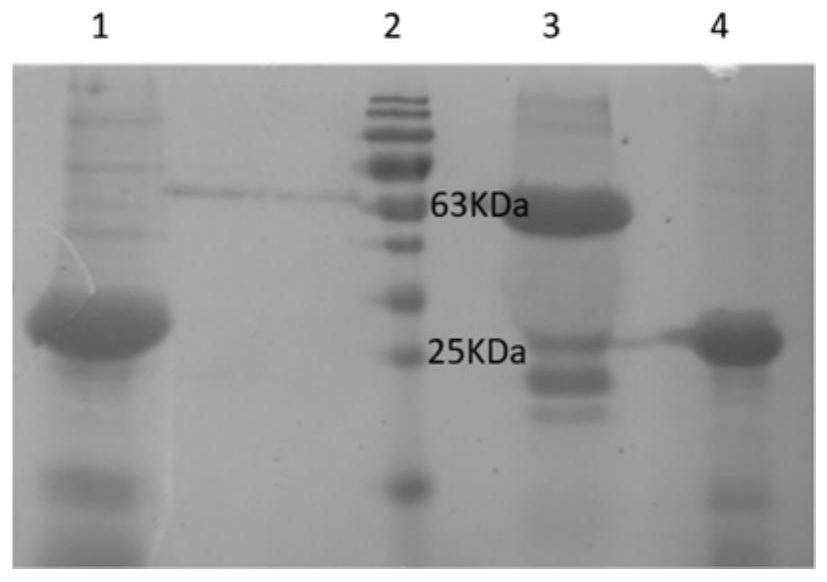
[0045] Example 1. Preparation of fusion protein
[0046] gene synthesis
[0047] 1. The fusion gene nmh encoding the fusion protein NMH has a base sequence as in SEQ ID NO:1 in Table 1, wherein the amino acid sequence of the NP fragment is as shown in SEQ ID NO:3, and its encoding gene np has The base sequence shown in the 1st to 90th positions of the sequence shown in SEQ ID NO:1. The amino acid sequence of M2e is shown in SEQ ID NO:4, and its coding gene m2e has the base sequence shown in the 111th to 248th positions of SEQ ID NO:1. The amino acid sequence of the HA2 fragment is shown in SEQ ID NO:5, and its coding gene ha2 has the base sequence shown in the 270th to 807th positions of SEQ ID NO:1. The base sequence of the DNA linker GSAGSAG (SEQ ID NO:6) encoding the short peptide is located at the 91st to 110th, 249th to 269th, 435th to 455th of the sequence shown in SEQ ID NO:1 bits and bits 621 to 641.
[0048] Table 1
[0049]
[0050] Note: The sequence in bo...
Embodiment 2
[0113] Example 2. Antibody Titer Research
[0114] Serotype study of subunit influenza vaccine immunized mice. Female BALB / c mice of 4-6 weeks were selected as research objects, and the same molar vaccine protein (NMH, NMHC, PBS (control)) was intraperitoneally immunized on day 0, day 14, and day 28 respectively, and the immune volume was 500 μl / mouse, 14 days after the third immunization (Day 42), the serum was collected for antibody research in the serum.
[0115] The hemagglutinin protein (HA) of six influenza subtypes including H1N1, H3N2, H2N2, H5N1, H7N9, and H9N2 (due to the limitation of experimental conditions, only the above six proteins were represented) was coated at a concentration of 2 μg / mL on the On the ELISA plate, wash the plate 4 times with PBST, block with blocking solution, wash the plate 4 times with PBST, and then incubate with the serially diluted sera of each immune group at 37°C for 1 hour, wash the plate 4 times with PBST, and incubate the seconda...
Embodiment 3
[0120] Embodiment 3. Trace neutralization experiment
[0121] On the first day, dilute the sera of different immune groups to the corresponding multiples in a 2-fold ratio, add 50 μl / well to a 96-well plate, and then add 50 μl / well 100TCID 50 The viruses (represented by H1N1 and H3N2 strains) were co-incubated at 37°C for 2 hours, and 100 μl / well MDCK (1.5*10^4, purchased from ATCC CCL-34 (MDCK (NBL-2))) was added to Incubate at 37°C for 20 hours, and set up a control group at the same time, positive control 50 μl virus dilution + 50 μl virus + 100 μl MDCK. Negative control 100 μl virus diluent + 100 μl MDCK. The next day, use ELISA to detect virus-infected cells, first use the mouse monoclonal antibody against the NP protein of influenza A (article number: 11675-T62-50, Beijing Yiqiao Shenzhou Technology Co., Ltd.) to detect the virus in MDCK cells In order to reflect the neutralization effect of the serum sample on a certain virus, the average OD value of the cell positi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com