Application of flavone derivative
A technology of derivatives and flavones, which is applied in the application field of flavone derivatives as FXR ligand drugs, can solve the problems of not being FXR antagonists and restricting use, and achieve the effect of wide indications and precise targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
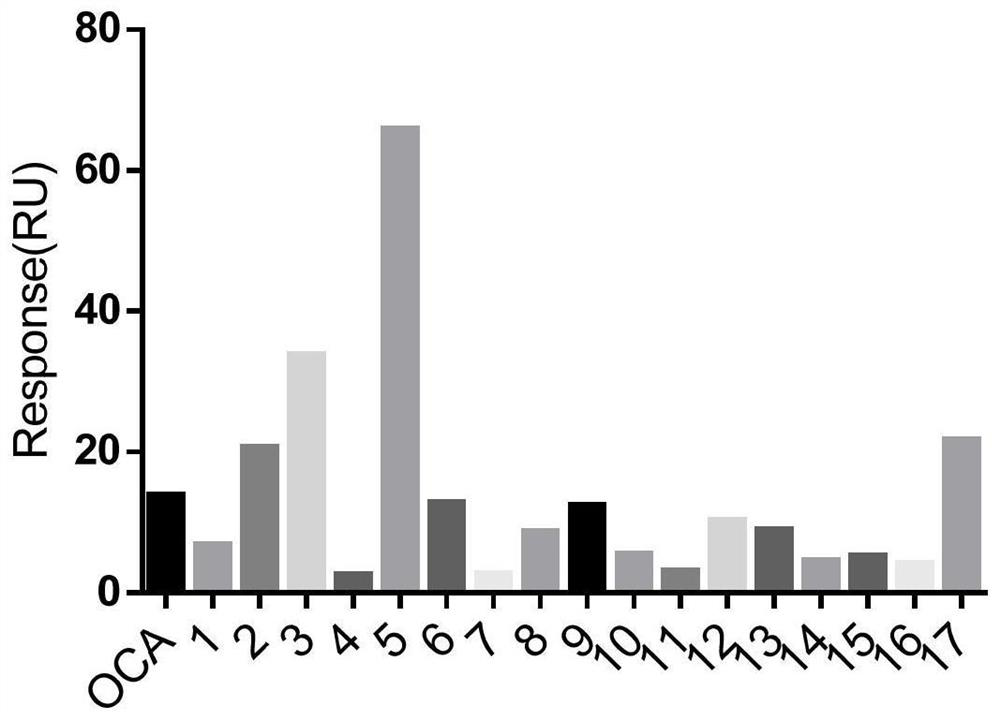
[0045] Example 1: FXR ligand screening based on surface plasmon resonance (SPR) molecular interaction technology
[0046] 1. Experimental materials
[0047] FXR recombinant protein was purchased from Sangon Bioengineering (Shanghai); all tested compounds were purchased from MCE; CM5 chip and sodium acetate solution were purchased from GE Healthcare; PBST was prepared according to the standard buffer formula.
[0048] 2. Experimental method
[0049] 2.1 Protein pre-enrichment
[0050] The protein was diluted to 20 μg / ml with sodium acetate pH 5.5, 5.0, 4.5 and 4.0 respectively, and 100 μL was prepared for each, and pH 4.0 was determined as the optimal coupling condition through pre-enrichment experiments. Therefore, the ligand solution was diluted to 20 μg / ml with sodium acetate at pH 4.0, and 200 μL was used for the formal coupling operation.
[0051] 2.2 Sample detection
[0052] The running buffer for small molecule samples was PBST containing 5% DMSO. Dilute the small ...
Embodiment 2
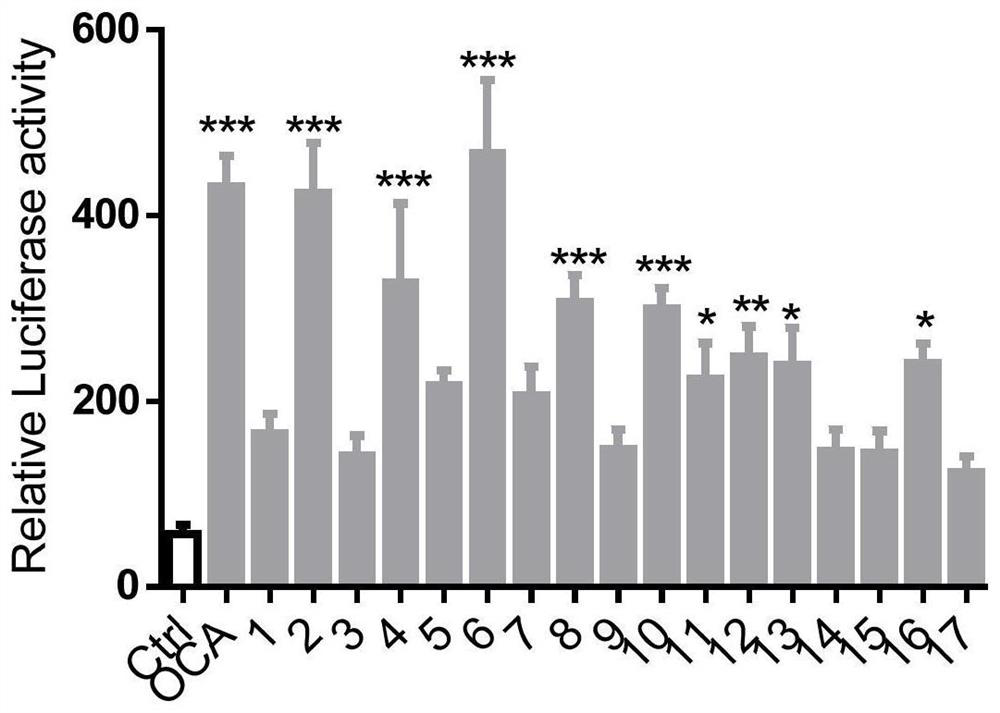
[0057] Example 2: Verification of FXR transcriptional activity based on dual luciferase reporter genes
[0058] 1. Experimental materials
[0059] AML12 cell line was purchased from Shanghai Cell Bank; Lipofectamine 3000 liposome was purchased from Invitrogen; Opti-MEM medium was purchased from Gibco; dual luciferase reporter gene kit was purchased from Vazyme; all tested compounds were purchased from MCE.
[0060] 2. Experimental method
[0061] 2.1 FXR plasmid transfection
[0062] FXR plasmid transfection Transfection was performed according to the Lipofectamine 3000 standard transfection protocol.
[0063] 2.1.1 Cell Seeding
[0064] The day before transfection, AML12 cells were inoculated with 8×10 5 The cell density was seeded in a six-well plate, placed at 37 ° C, 5% CO 2 , cultivated in an environment with a relative humidity of 90%. Cell density needs to grow to 70%-80% for transfection.
[0065] 2.1.2 Plasmid transfection
[0066] a) 0.5ml opti-mem+8μl lipo30...
Embodiment 3
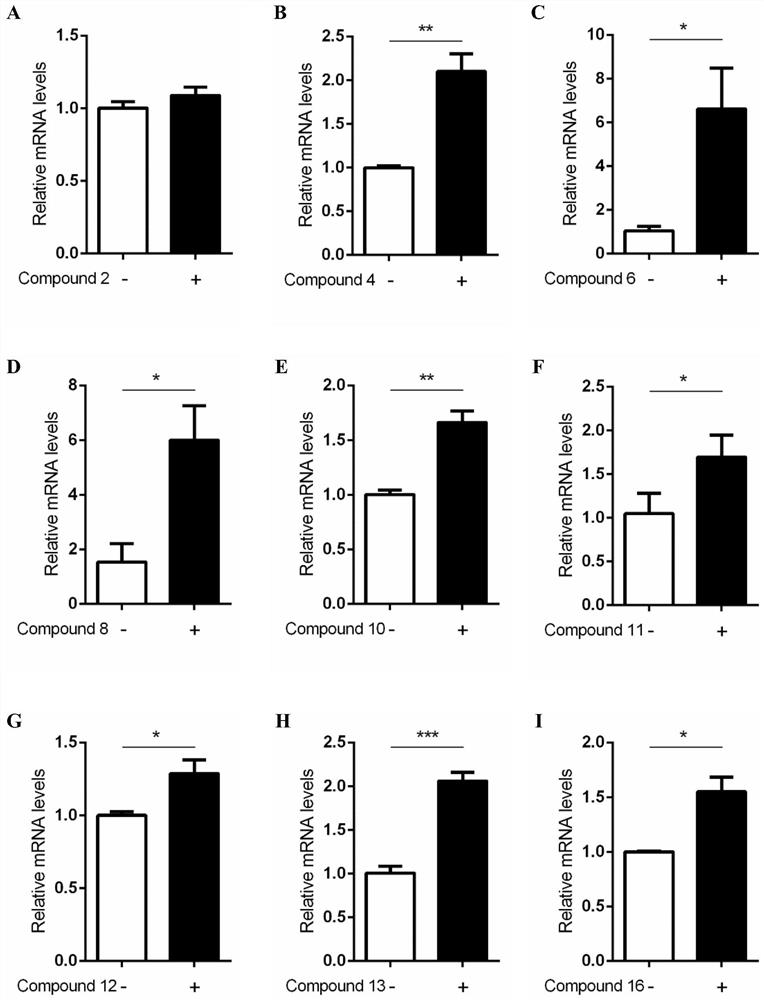
[0080] Example 3: Research on the regulation of FXR target genes by ligand compounds based on qRT-PCR
[0081] 1. Experimental materials
[0082] RNAiso Plus was purchased from TAKARA; reverse transcription reagents were purchased from Vazyme.
[0083] 2. Experimental method
[0084] The flavonoid Baicalein, which binds to FXR protein but has no agonistic effect, was administered to AML12 cells at a concentration of 10 μM and combined with OCA at a concentration of 1 μM for 12 hours, and then the cells were harvested.
[0085] 2.1 Extraction of Total RNA
[0086] After washing the cells with 1ml PBS, add 800μl RNAiso Plus to each well, and transfer the cells to a centrifuge tube by pipetting. Add 160 μl of chloroform (1 / 5 of the volume of RNAiso Plus) to the above lysate, close the cap of the centrifuge tube tightly, shake vigorously for 10 s, and after the mixture is fully emulsified, let it stand at room temperature for 10 min, and centrifuge at 12,000 rpm for 15 min at 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com